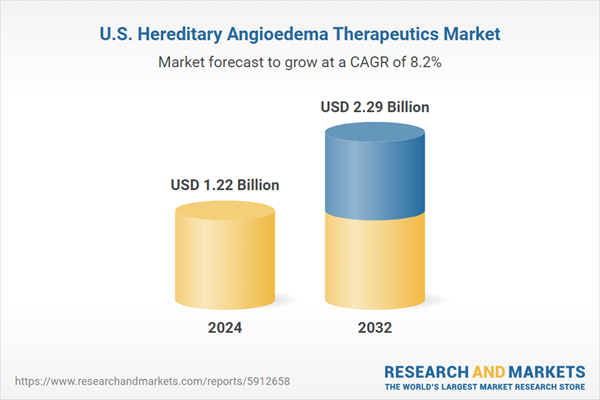
United States Hereditary Angioedema Therapeutics Market Analysis
Hereditary Angioedema (HAE) is a rare genetic disorder characterised by recurrent episodes of severe swelling in various parts of the body, including the extremities, face, gastrointestinal tract, and airway. The condition is caused by a deficiency or dysfunction of the C1 inhibitor protein, which plays a crucial role in regulating inflammation. The United States market for hereditary angioedema therapeutics has witnessed significant growth due to advancements in medical research, increased awareness, and the development of innovative treatments.Market Drivers
- Advancements in Biotechnology: The development of advanced biotechnological techniques has led to the introduction of novel therapies for HAE. These include C1 inhibitors, bradykinin receptor antagonists, and kallikrein inhibitors, which offer targeted treatment options and have improved patient outcomes.
- Increased Awareness and Diagnosis: Greater awareness among healthcare providers and the general public has led to earlier and more accurate diagnosis of HAE. This has resulted in an increase in the number of patients receiving appropriate treatment, thereby driving market growth.
- Favourable Regulatory Environment: The US Food and Drug Administration (FDA) has provided expedited approval pathways for rare disease treatments, including HAE therapeutics. This has encouraged pharmaceutical companies to invest in the development of new drugs, boosting market growth.
- Rising Healthcare Expenditure: The overall increase in healthcare expenditure in the United States has facilitated access to advanced treatments for rare diseases like HAE. This trend is expected to continue, further driving the market.
Challenges
- High Cost of Treatment: The cost of HAE therapeutics can be prohibitively high, posing a significant challenge for patients and healthcare systems. This is especially true for new biologic treatments, which can cost thousands of dollars per dose.
- Limited Patient Population: As a rare disease, HAE affects a relatively small number of individuals. This limits the potential market size and can deter pharmaceutical companies from investing heavily in research and development.
- Side Effects and Adverse Reactions: Some HAE treatments are associated with side effects and adverse reactions, which can impact patient compliance and overall treatment efficacy. Managing these side effects requires careful monitoring and can add to the overall cost of treatment.
- Complexity of Diagnosis: Despite increased awareness, diagnosing HAE can still be challenging due to its rarity and the similarity of its symptoms to other conditions. Misdiagnosis or delayed diagnosis can hinder timely treatment and affect patient outcomes.
Future Opportunities
- Development of Gene Therapies: Advances in gene therapy hold promise for providing long-term or even curative treatments for HAE. Research in this area is ongoing, and successful development could revolutionise the treatment landscape for HAE.
- Expansion of Treatment Options: Continued research and development efforts are likely to yield new and improved treatment options for HAE. These may include next-generation biologics, oral therapies, and combination treatments that offer enhanced efficacy and convenience.
- Personalised Medicine: The growing field of personalised medicine offers the potential for tailored treatments based on individual patient profiles. This approach could optimise treatment outcomes and minimise side effects, improving the overall quality of life for HAE patients.
- Increased Focus on Prophylactic Treatments: There is a growing emphasis on prophylactic (preventive) treatments for HAE, which aim to reduce the frequency and severity of attacks. This shift towards prevention could significantly improve patient outcomes and reduce the long-term burden of the disease.
- Collaboration and Partnerships: Increased collaboration between pharmaceutical companies, research institutions, and patient advocacy groups can accelerate the development of new treatments and improve access to existing therapies. Public-private partnerships and funding initiatives can play a crucial role in this process.
United States Hereditary Angioedema Therapeutics Market Trends
Hereditary Angioedema (HAE) is a rare genetic disorder marked by episodic swelling in various body parts, leading to significant morbidity. The United States market for HAE therapeutics is evolving rapidly, driven by scientific advancements and increased patient awareness.- Shift Towards Prophylactic Therapies: There is a noticeable trend towards the development and adoption of prophylactic treatments aimed at preventing HAE attacks rather than treating them reactively. These therapies, including newer long-acting C1 inhibitors, offer patients better control over their condition and improve their quality of life.
- Increased Adoption of Subcutaneous Administration: Subcutaneous formulations of HAE treatments are gaining popularity due to their ease of administration compared to intravenous options. This trend is driven by the convenience of at-home treatment, which reduces the need for frequent hospital visits and empowers patients with more autonomy.
- Integration of Digital Health Solutions: The integration of digital health technologies, such as mobile apps and wearable devices, is enhancing disease management for HAE patients. These tools assist in monitoring symptoms, tracking treatment adherence, and providing real-time data to healthcare providers, leading to more personalised and effective care.
- Focus on Paediatric Treatment Options: With increasing awareness of HAE in children, there is a growing focus on developing paediatric-specific treatments. This includes age-appropriate dosing and formulations that are safe and effective for younger patients, addressing a previously underserved segment of the market.
- Expansion of Patient Support Programs: Pharmaceutical companies are increasingly investing in comprehensive patient support programs. These programs offer financial assistance, education, and counselling services to help patients navigate the complexities of HAE treatment, thereby improving adherence and outcomes.
- Regulatory Incentives and Orphan Drug Designations: The FDA’s incentives for orphan drug development, including market exclusivity and tax credits, are encouraging more companies to invest in HAE therapeutics. This regulatory support is fostering innovation and expanding the range of available treatments.
- Rising Interest in Gene Therapy: Gene therapy is emerging as a promising area of research for HAE. Advances in this field could potentially offer curative treatments by correcting the underlying genetic defects, providing a one-time solution for patients.
United States Hereditary Angioedema Therapeutics Market Segmentation
Market Breakup by Drug Type
- C1-Esterase Inhibitor
- Bradykinin B2 Receptor Antagonist
- Kallikrein Inhibitor
- Others
Market Breakup by Route of Administration
- Parenteral
- Oral
United States Hereditary Angioedema Therapeutics Market Competitive Landscape
The United States hereditary angioedema therapeutics market is highly competitive, with key players including Takeda Pharmaceutical Company Limited, BioCryst Pharmaceuticals, Inc., Sanofi, CSL Behring LLC, Pharming Group N.V., Cipla, Inc., Ionis Pharmaceuticals Inc., Attune Pharmaceuticals, Inc., Adverum Biotechnologies, Inc., Arrowhead Pharmaceuticals, Inc., and Pharvaris B.V.Common market activities include mergers and acquisitions to enhance product portfolios and expand market reach. Companies are actively engaging in research initiatives to develop innovative therapies and improve existing treatments. Frequent product introductions highlight the focus on addressing unmet medical needs and improving patient outcomes. Strategic partnerships and collaborations are also prevalent, aimed at leveraging combined expertise and resources to accelerate development and commercialisation efforts. These activities collectively drive market growth and enhance competitive positioning in the hereditary angioedema therapeutics market.
Key Questions Answered in the Report
- What is the current and future performance of the United States hereditary angioedema therapeutics market?
- What are the main challenges facing the United States hereditary angioedema therapeutics market?
- What are the key drivers of the United States hereditary angioedema therapeutics market?
- What emerging trends are shaping the future of the United States hereditary angioedema therapeutics market?
- How have advanced biotechnological techniques impacted the development of novel therapies for HAE?
- How do digital health technologies improve disease management and care for HAE patients?
- How are FDA incentives for orphan drug development driving innovation in HAE therapeutics?
- How are novel formulations of bradykinin B2 receptor antagonists and kallikrein inhibitors improving patient compliance?
- What are the common strategies used by key players in the United States hereditary angioedema therapeutics market?
Key Benefits for Stakeholders
- The industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the United States hereditary angioedema therapeutics market from 2017-2032.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the United States hereditary angioedema therapeutics market.
- The study maps the leading, as well as the fastest-growing, regional markets. It further enables stakeholders to identify the key country-level markets within each region.
- Porter's five forces analysis assists stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the United States hereditary angioedema therapeutics industry and its attractiveness.
- The competitive landscape allows stakeholders to understand their competitive environment and provides insight into the current positions of key players in the market.
This product will be delivered within 5-7 business days.
Table of Contents
Companies Mentioned
- Takeda Pharmaceutical Company Limited
- BioCryst Pharmaceuticals, Inc.
- Sanofi
- CSL Behring LLC.
- Pharming Group N.V.
- Cipla, Inc.
- Ionis Pharmaceuticals Inc.
- Attune Pharmaceuticals, Inc.
- Adverum Biotechnologies, Inc.
- Arrowhead Pharmaceuticals, Inc.
- Pharvaris B.V
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | August 2024 |
| Forecast Period | 2024 - 2032 |
| Estimated Market Value ( USD | $ 1.22 Billion |
| Forecasted Market Value ( USD | $ 2.29 Billion |
| Compound Annual Growth Rate | 8.2% |
| Regions Covered | United States |
| No. of Companies Mentioned | 11 |