Global Biocompatible Materials Market - Key Trends and Drivers Summarized
What Are Biocompatible Materials and How Are They Revolutionizing Medicine?
Biocompatible materials are engineered to interact with the human body in ways that are not harmful, making them integral to the success of medical implants, devices, and prosthetics. These materials must meet rigorous safety standards, ensuring that they perform their intended functions without causing adverse immune reactions, infections, or toxicity. Common biocompatible materials include certain polymers, metals, ceramics, and natural substances that can be adapted for medical applications. For instance, titanium is widely used for bone implants and joint replacements due to its strength, durability, and high biocompatibility, as it does not corrode inside the body. Similarly, silicone rubber is favored for implants and catheters because of its flexibility and inert properties. The development of biocompatible materials is driven by the need to enhance the longevity and functionality of medical devices and improve the quality of life for patients.How Are Advanced Technologies Enhancing Biocompatible Materials?
The field of biocompatible materials is advancing rapidly, thanks to breakthroughs in nanotechnology, biotechnology, and material science. Researchers are now able to design materials at the molecular level, tailoring their properties to specific medical applications. For example, hydrogels made from polymers that can absorb significant amounts of water are being used to create soft contact lenses and wound dressings that promote healing. Additionally, surface modification techniques allow scientists to enhance the interaction between the material and the biological environment, improving the integration of implants with body tissues. Innovations such as biodegradable materials also show promise for reducing the long-term risk associated with implants, as these materials can gradually dissolve and be absorbed or excreted by the body, eliminating the need for surgical removal after they have served their purpose.What Challenges Do Biocompatible Materials Face in Clinical Applications?
While biocompatible materials have transformative potential, their clinical application comes with several challenges. The complexity of human biology means that predicting how materials will behave inside the body can be incredibly complex. Immune response, bio-corrosion, and long-term stability are among the significant concerns that researchers must address. For example, metal ions from corroding implants can accumulate in the body and potentially lead to metal toxicity or allergic reactions. Furthermore, the cost of developing and testing new biocompatible materials can be prohibitively high, often necessitating extensive preclinical and clinical trials to ensure they meet safety and efficacy standards. These challenges require ongoing research and collaboration between material scientists, engineers, and medical professionals to ensure that new developments can be safely and effectively translated into clinical practice.What Drives the Growth in the Biocompatible Materials Market?
The growth in the biocompatible materials market is driven by several factors, including technological advancements that allow for the development of more sophisticated and effective products. An aging global population and a corresponding increase in the prevalence of chronic diseases such as osteoarthritis and cardiovascular diseases are also significant growth drivers, as these conditions often require long-term implantable devices and surgical interventions. Additionally, the increasing demand for minimally invasive surgeries promotes the use of biocompatible materials that can be delivered through smaller incisions and offer quicker recovery times and reduced risk of infection. Consumer behavior is also evolving, with patients increasingly seeking treatments that improve not only longevity but also quality of life, which biocompatible materials can offer. Moreover, regulatory support for biocompatible materials in emerging markets is expanding access to novel medical technologies, further stimulating growth in the industry. These factors collectively ensure that the market for biocompatible materials continues to expand, providing essential solutions for a broad range of medical applications.Report Scope
The report analyzes the Biocompatible Materials market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Material Type (Polymeric, Metallic, Ceramic, Other Material Types); Application (Orthopedic, Cardiovascular, Dental, Neurology, Ophthalmology, Other Applications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Polymeric Materials segment, which is expected to reach US$231 Billion by 2030 with a CAGR of 16.9%. The Metallic Materials segment is also set to grow at 14.3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $56.7 Billion in 2024, and China, forecasted to grow at an impressive 14.4% CAGR to reach $74.8 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Biocompatible Materials Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Biocompatible Materials Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Biocompatible Materials Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AdvanSource Biomaterials Corporation, BASF SE, Celanese Corporation, Collagen Solutions Plc, Corbion N.V and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 53 companies featured in this Biocompatible Materials market report include:
- AdvanSource Biomaterials Corporation
- BASF SE
- Celanese Corporation
- Collagen Solutions Plc
- Corbion N.V
- Exactech
- Morgan Technical Ceramics
- Nobel Biomaterials
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AdvanSource Biomaterials Corporation
- BASF SE
- Celanese Corporation
- Collagen Solutions Plc
- Corbion N.V
- Exactech
- Morgan Technical Ceramics
- Nobel Biomaterials
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 199 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
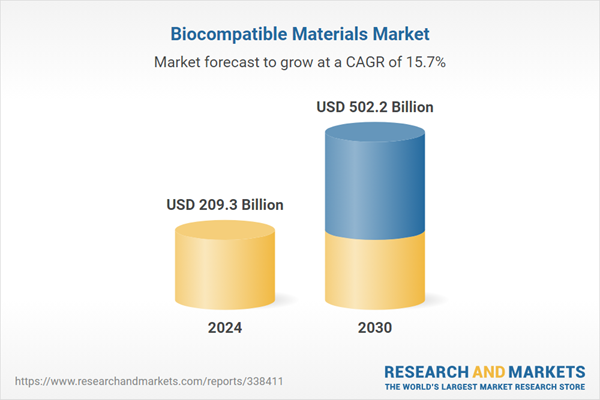
| Estimated Market Value ( USD | $ 209.3 Billion |
| Forecasted Market Value ( USD | $ 502.2 Billion |
| Compound Annual Growth Rate | 15.7% |
| Regions Covered | Global |