The COVID-19 pandemic impacted routine sexually transmitted infection services, suggesting an increase in syndromic sexually transmitted infections and missing asymptomatic cases. The pandemic also had a major impact on the gonorrhea therapeutics market. For instance, an article published in the February 2022 issue of the journal AANP reported that testing and treatment algorithms for sexually transmitted diseases (STDs) like gonorrhea were impacted during the pandemic due to a decrease in staffing coupled with reduced diagnostic capability and disturbances in the supply chain that led to a decrease or delay in gonorrhea diagnosis and treatment that led to an increase in gonorrhea. Thus, the COVID-19 pandemic impacted the gonorrhea therapeutics market. However, in the current scenario, with the decrease in COVID-19 cases and the resumption of healthcare services, the market is expected to witness significant growth over the forecast period.
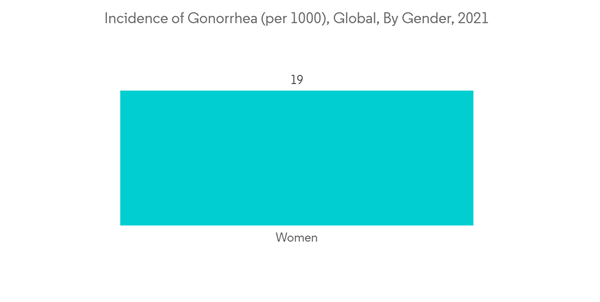
The factors that are driving this market are the growing burden of gonorrhea and growing government and private sector participation through national campaigns. The increased number of gonorrhea cases worldwide is leading to a demand for better therapeutics, thereby driving the demand for gonorrhea therapeutics. For instance, in August 2022, WHO reported that in 2020 there were 82.4 million new cases of HIV infection among adolescents and adults aged 15-49 years worldwide, with a global incident rate of 19 per 1000 women and 23 per 1000 men. Most cases were in the African Region and the Western Pacific Region. Thus, increasing gonorrhea cases are increasing the demand for gonorrhea therapeutics and thus driving the demand for this market.
Moreover, the growing government and private sector participation in national campaigns is also fueling the growth of the studied market. For instance, in August 2022, WHO reported that the Global Health Sector Strategy on HIV, Hepatitis, and STIs (2022-2030) has set targets to reduce the number of new cases of gonorrhea among people 15-49 years old from 82.3 million per year in 2020 to 8.23 million per year in 2030, thus reducing the annual incidence by 90% by 2030. The effective prevention and control of gonococcal infections by using prevention messages, interventions, and the right treatment plans.
Similarly, in June 2022, the Australian Government, Department of Health and Aged Care, stated some fundamental guidelines to prevent gonorrhea that included knowledge of experienced professionals and providing guidance on best practices to treat gonorrhea. So, these government programs make people more aware, which increases the demand for government therapies and helps the studied market grow.
Thus, due to the growing burden of gonorrhea and growing government and private sector participation through national campaigns, the market is expected to witness significant growth over the forecast period. But strict regulations and the social stigma that comes with people going to STD clinics could slow the growth of the market that was studied.
Gonorrhea Therapeutics Market Trends
Dual Therapy Segment is Expected to Register a Significant Growth Over the Forecast Period.
The dual therapy segment is expected to witness significant growth over the forecast period owing to the increasing number of gonorrhea cases caused by Neisseria gonorrhoeae and the efficacy of dual therapy in the treatment of this disease. Dual therapy with ceftriaxone and azithromycin is recommended as the first-line regimen for the treatment of gonorrhea. Also, in 2021, WHO will recommend dual therapy over monotherapy for people with symptomatic and asymptomatic gonorrhea. Thus, the increasing use of dual therapy treatments is driving the growth of this segment.Many clinical studies and research have also concluded that dual therapy is more effective in gonorrhea treatment as compared to monotherapy. For example, an article published in the November 2021 issue of the Journal of Infectious Diseases reported that the European Union against Sexually Transmitted Infections had implemented dual therapy for gonorrhea (azithromycin and ceftriaxone) for gonorrhea treatment.
Similarly, another article published in the journal Clinical Infectious Diseases in October 2021 reported the efficacy of dual therapy for the treatment of gonorrhea infections. The article also quoted the evidence on the efficacy of high-dose ceftriaxone monotherapy for extragenital Neisseria gonorrhoeae (NG) infection. Thus, increasing the efficacy of dual therapy in the treatment of gonorrhea is driving the growth of this segment.
Thus, the increasing efficacy of dual therapy for gonorrhea treatment is driving the growth of this segment over the forecast period, thereby driving the market.
North America is Estimated to Register a Significant Growth Over the Forecast Period.
The North American region is expected to witness significant growth over the forecast period owing to the high standards of healthcare infrastructure and the easy availability of sophisticated technology in this region. As per CDC estimates, in September 2022, the Center for Disease Control and Prevention (CDC) reported that in 2021, in the United States, 6,94,796 cases of gonorrhea were reported, and this number increased by 25.4% in the last four years. The United States reported a higher number of gonorrhea cases in men than in women.Further, recent developments such as mergers, acquisitions, product launches, and partnerships among key market players are also propelling the growth of the studied market. In March 2022, Evofem Biosciences, Inc. announced that it had completed enrollment in EVOGUARD, the registrational Phase 3 clinical trial evaluating EVO100 for two potential new indications: the prevention of chlamydia infection in women and the prevention of urogenital gonorrhea infection in women. Thus, the search for new product launches provides better therapeutics to patients, contributing to the growth of the region's studied market.
Similarly, the government initiatives in the region for the effective control of STDs like gonorrhea are also driving the growth of the studied market. For instance, in August 2022, the American Medical Association launched the creation of a toolkit for the nation's physicians to promote screening of STIs that included HIV, gonorrhea, viral hepatitis, and latent tuberculosis. Also, in July 2022, the National Strategic Plan aims to reverse the recent dramatic rise in STIs in the United States. The STI plan aims to provide a roadmap for a broad range of stakeholders, including public health, health care, government, community-based organizations, educational institutions, researchers, private industry, and academia, to develop, enhance, and expand STI prevention and care programs at the local, state, tribal, and national levels over the next five years. The diseases covered in this plan include secondary syphilis, congenital syphilis, gonorrhea, human papillomavirus, chlamydia, etc.
Thus, due to the high standards of healthcare infrastructure and the easy availability of sophisticated technology and technological developments, the region is expected to witness significant growth over the forecast period.
Gonorrhea Therapeutics Market Competitor Analysis
The market for gonorrhea therapeutics is fragmented in terms of competition, despite the presence of major pharmaceutical companies. Some of the major players in the gonorrhea therapeutics market include AbbVie (Allergan, Inc.), F. Hoffmann-La Roche Ltd., GlaxoSmithKline plc, Lupin Pharmaceuticals, Pfizer Inc., Teligent, Inc., Merck & Co., Inc., Hologic, Inc., Danaher Corporation, Becton Dickinson and Company, and AstraZeneca.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie (Allergan, Inc.)
- F. Hoffmann-La Roche Ltd
- GlaxoSmithKline plc
- Lupin Pharmaceuticals
- Pfizer Inc.
- Teligent, Inc.
- Merck & Co., Inc.
- Hologic, Inc.
- Danaher Corporation
- Abbott
- Becton Dickinson and Company
- AstraZeneca