Global Intranasal Drug Delivery Market - Key Trends and Drivers Summarized
Intranasal drug delivery refers to the administration of medications through the nasal cavity, offering a non-invasive and efficient route for drug absorption. This method leverages the rich vascularization and large surface area of the nasal mucosa, enabling rapid onset of action and enhanced bioavailability compared to other non-invasive routes. Intranasal delivery systems are utilized for a variety of drugs, including those for pain management, neurological disorders, and vaccinations. The technology behind these systems includes metered-dose nasal sprays, nasal gels, and powder formulations, each designed to optimize drug absorption and minimize systemic side effects.The application of intranasal drug delivery spans several therapeutic areas, making it a versatile tool in modern medicine. It is particularly beneficial in emergency situations where rapid drug action is critical, such as with naloxone for opioid overdoses or epinephrine for anaphylactic reactions. Additionally, intranasal delivery is increasingly used for chronic conditions like migraines and hormonal therapies, where it offers a convenient and non-invasive alternative to injections. The ability to bypass the gastrointestinal tract and first-pass metabolism in the liver also makes this route advantageous for drugs that are poorly absorbed orally or extensively metabolized.
The growth in the intranasal drug delivery market is driven by several factors, including advancements in delivery technologies, increasing prevalence of chronic and acute conditions requiring rapid intervention, and a growing preference for non-invasive administration methods among patients and healthcare providers. Technological innovations, such as improved formulations and novel delivery devices, enhance drug stability and absorption, expanding the range of treatable conditions. Additionally, demographic trends such as aging populations and rising incidences of conditions like Alzheimer's disease and Parkinson's disease fuel demand for more effective drug delivery systems. Consumer behavior shifts towards self-administration and home care, coupled with healthcare cost containment strategies, further propel market expansion. The development of intranasal vaccines, particularly highlighted by the COVID-19 pandemic, also underscores the critical role of this delivery method in global health strategies.
Report Scope
The report analyzes the Intranasal Drug Delivery market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Product Type (Liquid Delivery Devices, Powder Delivery Devices, Pressurized Metered Dose Inhalers, Other Product Types); Application (Respiratory Disorders, Neurological Disorders, Pain Management, Other Applications).
- Geographic Regions/Countries: World; USA; Canada; Japan; China; Europe; France; Germany; Italy; UK; Spain; Russia; Rest of Europe; Asia-Pacific; Australia; India; South Korea; Rest of Asia-Pacific; Latin America; Argentina; Brazil; Mexico; Rest of Latin America; Middle East; Iran; Israel; Saudi Arabia; UAE; Rest of Middle East; Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Liquid Delivery Devices segment, which is expected to reach US$38.2 Billion by 2030 with a CAGR of 6.3%. The Powder Delivery Devices segment is also set to grow at 7.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $26.2 Billion in 2024, and China, forecasted to grow at an impressive 9.1% CAGR to reach $8.2 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Intranasal Drug Delivery Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Intranasal Drug Delivery Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Intranasal Drug Delivery Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Acorda Therapeutics, Inc., Ferring Pharmaceuticals Inc, Bespak Europe Ltd., Breckenridge Pharmaceutical, Inc., Janssen Global Services LLC and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 77 companies featured in this Intranasal Drug Delivery market report include:
- Acorda Therapeutics, Inc.
- Ferring Pharmaceuticals Inc
- Bespak Europe Ltd.
- Breckenridge Pharmaceutical, Inc.
- Janssen Global Services LLC
- Hikma Pharmaceuticals PLC
- Biologische Heilmittel Heel GmbH
- Evoke Pharma, Inc.
- KemPharm, Inc.
- Impel NeuroPharma
- Actiza Pharmaceutical Pvt., Ltd.
- Covis Pharma
- H&T Presspart Manufacturing Ltd.
- Biomedica International
- Alchemy Pharmatech Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Acorda Therapeutics, Inc.
- Ferring Pharmaceuticals Inc
- Bespak Europe Ltd.
- Breckenridge Pharmaceutical, Inc.
- Janssen Global Services LLC
- Hikma Pharmaceuticals PLC
- Biologische Heilmittel Heel GmbH
- Evoke Pharma, Inc.
- KemPharm, Inc.
- Impel NeuroPharma
- Actiza Pharmaceutical Pvt., Ltd.
- Covis Pharma
- H&T Presspart Manufacturing Ltd.
- Biomedica International
- Alchemy Pharmatech Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 320 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
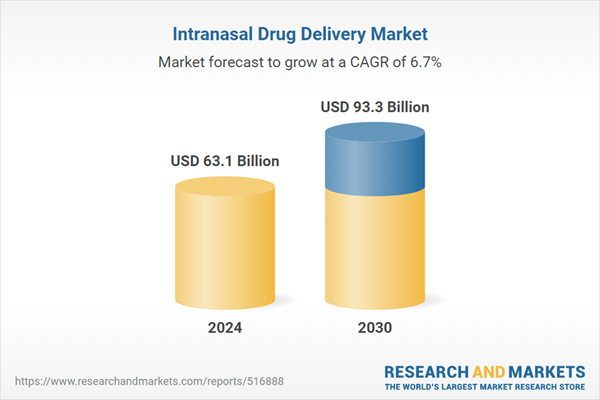
| Estimated Market Value ( USD | $ 63.1 Billion |
| Forecasted Market Value ( USD | $ 93.3 Billion |
| Compound Annual Growth Rate | 6.7% |
| Regions Covered | Global |