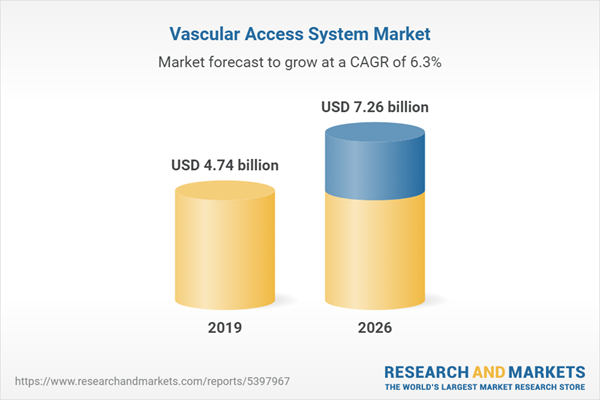
The global vascular access systems market is evaluated at US$ 4.744 billion for the year 2019 growing at a CAGR of 6.27% reaching the market size of US$ 7.259 billion by the year 2026. Venous or arterial access is obtained through vascular access systems. Vascular Access Devices are medical equipment used to get ingress the bloodstream to supply medicines, administer fluids, nutritional components, and collection of blood vessels and their products. These vascular access tubes are made from latex, silicon, and other substances. The procedure involves placing rigid plastic tubes into blood vessels such as the basilic vein and subclavian vein to perform diagnostic tests and treatments such as chemotherapy, intravenous antibiotic treatment, and intravenous feeding. It includes blood sampling, blood transfusion, fluid infusion, and central venous pressure readings. The vascular access catheter devices are also significantly used in the case of chemotherapies.
The blood is obtained from or delivered to a person’s bloodstream over a continuous period through different vascular access devices such as cannulae, catheters, peripherals, and ports. The procedure relieves patients from the continuous stress of needles and offers a painless method to draw blood or provide medication. The rising global prevalence of diseases such as kidney failure, diabetes, and cancer which involve the transfer of blood to and from the human body is positively impacting the demand for vascular access systems. High infection risk associated with vascular access devices and stringent government approval regulations restrains the growth of the vascular access systems market. Over the past decade, there has been a rise in the prevalence of several chronic diseases that need vascular access treatment. Some of the major chronic diseases include cancer, kidney failure, and heart diseases, among others. Along with these, there are several lifestyle disorders relating to chronic and non-communicable disease conditions, such as hypertension, diabetes, obesity, and depression, among others, which need critical care during their hospitalization.
The prevalence of end-stage renal disease (ESRD), worldwide, is further increasing at a significant rate, resulting in chronic kidney disease (CKD).
In 2017, in the United States, around 30 million people (15% of US adults) were found suffering from chronic kidney disease (CKD). CKD was found to be more common in women (16%) than in men (13%). For patients undergoing dialysis treatment, the ideal method of vascular access is through an arteriovenous fistula (ANF), by referring to a nephrologist about replacement therapy.
The prevalence of diabetes, increasing rates of road accidents, and an increasing number of surgical procedures are expected to drive the market. Increasing demand for advanced techniques, such as needleless administration of drugs is also a vital driver for this market. Every day many healthcare workers handle drugs in hospitals and other healthcare centers. Despite precaution measures availability, there remains a risk of exposure to these drugs during procedures. Such exposures may occur through, ingestion, absorption, accidental inhalation, or dermal contact of a minimal amount of these drugs which may lead to a variety of health problems.
The advent of COVID-19 had a positive impact on the market of vascular access systems as it became an integral part of the treatment procedures that were followed during the period to cure the patients. Moreover, WHO declared COVID-19 a pandemic as the increase in the number of cases was exponential in the initial months. The medical authorities advised the hospitals and other such medical institutions to stock up the requirements to never run out of supplies during the treatments, which in turn, increased the demand for vascular access systems in the year 2020.
Innovations in the sector have brought down the costs of these products in recent years and the trend is expected to continue during the forecast period.
The market has been shifting towards less expensive catheter devices that tend to have lower rates of failure. The midlines and extended dwell catheters are emerging as lower-cost alternatives which results in a downward force on market value. Both of these catheters are expected to continue growing substantially as clinicians continue to search for solutions that are less invasive than PICCs but provide a longer dwell time than PIVCs. Concerns about catheter-related infections and failures have been applying significant downward pressure on the vascular access market. Many central line catheters are associated with high infection rates, and treating any infections or complications caused by a catheter can be costly and time-consuming for the facility.
As such, clinician preference has trended towards alternative modes of access that have lower complication rates, such as using AV fistula instead of long-term dialysis catheters. As a result of concerns about catheter-related infections and complications, many new vascular access accessories are expected to grow rapidly. Vascular access visualization technologies, such as ultrasound, vein visualization, or tip-placement devices, are intended to bolster catheter performance by improving rates of successful placements, decreasing cases of dislodgment caused by improperly placed catheters, and reducing cases of needle stick injury by lowering the number of attempts required per catheter.
The blood is obtained from or delivered to a person’s bloodstream over a continuous period through different vascular access devices such as cannulae, catheters, peripherals, and ports. The procedure relieves patients from the continuous stress of needles and offers a painless method to draw blood or provide medication. The rising global prevalence of diseases such as kidney failure, diabetes, and cancer which involve the transfer of blood to and from the human body is positively impacting the demand for vascular access systems. High infection risk associated with vascular access devices and stringent government approval regulations restrains the growth of the vascular access systems market. Over the past decade, there has been a rise in the prevalence of several chronic diseases that need vascular access treatment. Some of the major chronic diseases include cancer, kidney failure, and heart diseases, among others. Along with these, there are several lifestyle disorders relating to chronic and non-communicable disease conditions, such as hypertension, diabetes, obesity, and depression, among others, which need critical care during their hospitalization.
The prevalence of end-stage renal disease (ESRD), worldwide, is further increasing at a significant rate, resulting in chronic kidney disease (CKD).
In 2017, in the United States, around 30 million people (15% of US adults) were found suffering from chronic kidney disease (CKD). CKD was found to be more common in women (16%) than in men (13%). For patients undergoing dialysis treatment, the ideal method of vascular access is through an arteriovenous fistula (ANF), by referring to a nephrologist about replacement therapy.
The prevalence of diabetes, increasing rates of road accidents, and an increasing number of surgical procedures are expected to drive the market. Increasing demand for advanced techniques, such as needleless administration of drugs is also a vital driver for this market. Every day many healthcare workers handle drugs in hospitals and other healthcare centers. Despite precaution measures availability, there remains a risk of exposure to these drugs during procedures. Such exposures may occur through, ingestion, absorption, accidental inhalation, or dermal contact of a minimal amount of these drugs which may lead to a variety of health problems.
The advent of COVID-19 had a positive impact on the market of vascular access systems as it became an integral part of the treatment procedures that were followed during the period to cure the patients. Moreover, WHO declared COVID-19 a pandemic as the increase in the number of cases was exponential in the initial months. The medical authorities advised the hospitals and other such medical institutions to stock up the requirements to never run out of supplies during the treatments, which in turn, increased the demand for vascular access systems in the year 2020.
Innovations in the sector have brought down the costs of these products in recent years and the trend is expected to continue during the forecast period.
The market has been shifting towards less expensive catheter devices that tend to have lower rates of failure. The midlines and extended dwell catheters are emerging as lower-cost alternatives which results in a downward force on market value. Both of these catheters are expected to continue growing substantially as clinicians continue to search for solutions that are less invasive than PICCs but provide a longer dwell time than PIVCs. Concerns about catheter-related infections and failures have been applying significant downward pressure on the vascular access market. Many central line catheters are associated with high infection rates, and treating any infections or complications caused by a catheter can be costly and time-consuming for the facility.
As such, clinician preference has trended towards alternative modes of access that have lower complication rates, such as using AV fistula instead of long-term dialysis catheters. As a result of concerns about catheter-related infections and complications, many new vascular access accessories are expected to grow rapidly. Vascular access visualization technologies, such as ultrasound, vein visualization, or tip-placement devices, are intended to bolster catheter performance by improving rates of successful placements, decreasing cases of dislodgment caused by improperly placed catheters, and reducing cases of needle stick injury by lowering the number of attempts required per catheter.
Market Segmentation:
By Product
- Peripherally Inserted Central Catheter (PICC)
- Non-Tunneled Central Catheter
- Tunneled Catheter
- Port Catheter
By Application
- Intravenous Antibiotic Treatment
- Blood Transfusion
- Long-Term IV Feeding
- Chemotherapy
- Others
By End User
- Hospitals and Clinics
- Ambulatory Surgical Centers
- Catheterization Labs
By Geography
- North America
- USA
- Canada
- Mexico
- South America
- Brazil
- Argentina
- Others
- Europe
- Germany
- France
- UK
- Others
- Middle East & Africa
- Saudi Arabia
- UAE
- Others
- Asia Pacific
- China
- India
- Japan
- South Korea
- Taiwan
- Thailand
- Indonesia
- Others
Table of Contents
1. Introduction
2. Research Methodology
3. Executive Summary
4. Market Dynamics
5. Vascular Access Systems Market Analysis, by Product
6. Vascular Access Systems Market Analysis, by Application
7. Vascular Access Systems Market Analysis, by End-user
8. Vascular Access Systems Market Analysis, by Geography
9. Competitive Environment and Analysis
10. Company Profiles
Companies Mentioned
- Edwards Lifesciences Corporation
- Baxter International Inc.
- C.R. Bard, Inc.
- Fresenius Medical Care AG & Co. KGaA
- Becton, Dickinson and Company
- Teleflex Incorporated
- Medtronic PLC
- B.Braun Melsungen AG
- Smiths Medical
- Biomerics
Methodology

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 122 |
| Published | July 2021 |
| Forecast Period | 2019 - 2026 |
| Estimated Market Value ( USD | $ 4.74 billion |
| Forecasted Market Value ( USD | $ 7.26 billion |
| Compound Annual Growth Rate | 6.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |