List of Tables
Summary Table A: Global Market for Companion Diagnostics, by Product, Through 2028
Summary Table B: Global Market for Companion Diagnostics, by Region, Through 2028
Table 1: Expanded/New CDx Testing for Personalized Medicine in 2022
Table 2: Global Market for Companion Diagnostics, by Product and Service, Through 2028
Table 3: Companion Diagnostics Instruments
Table 4: Global Market for Companion Diagnostics Instruments, by Region, Through 2028
Table 5: Global Market for Companion Diagnostics Consumables, by Region, Through 2028
Table 6: Global Market for Companion Diagnostics Services, by Region, Through 2028
Table 7: Global Market for Companion Diagnostics, by Type of Test, Through 2028
Table 8: Global Market for Laboratory Developed Test (LDT)-CDx, by Region, Through 2028
Table 9: List of FDA-Approved Companion Diagnostics, by Company, as of December 2023
Table 10: Global Market for Commercial-CDx, by Region, Through 2028
Table 11: FDA-Approved CDx Tests, by Technology, as of December 2023
Table 12: Global Market for Companion Diagnostics, by Technology, Through 2028
Table 13: List of FDA-Approved PCR-CDx, by Company, as of December 2023
Table 14: Global Market for Polymerase Chain Reaction CDx, by Region, Through 2028
Table 15: Global Market for Polymerase Chain Reaction CDx, by Application, Through 2028
Table 16: List of FDA-Approved IHC-CDx, by Company, as of December 2023
Table 17: Global Market for Immunohistochemistry CDx, by Region, Through 2028
Table 18: Global Market for Immunohistochemistry CDx, by Application, Through 2028
Table 19: List of FDA-Approved NGS-CDx, by Company, as of December 2023
Table 20: Global Market for Next-Generation Sequencing CDx, by Region, Through 2028
Table 21: Global Market for Next-Generation Sequencing CDx, by Application, Through 2028
Table 22: List of FDA-Approved ISH-CDx, by Company, as of December 2023
Table 23: Global Market for <i>In Situ</i> Hybridization CDx, by Region, Through 2028
Table 24: Global Market for In Situ Hybridization CDx, by Application, Through 2028
Table 25: Global Market for Genotyping CDx, by Region, Through 2028
Table 26: Global Market for Genotyping CDx, by Application, Through 2028
Table 27: Global Market for Immunoassays CDx, by Region, Through 2028
Table 28: Global Market for Immunoassays CDx, by Application, Through 2028
Table 29: Global Market for Exosome CDx, by Region, Through 2028
Table 30: Global Market for Exosomes CDx, by Application, Through 2028
Table 31: Global Market for Other CDx, by Region, Through 2028
Table 32: Global Market for Other CDx, by Application, Through 2028
Table 33: Technological Expertise in the CDx Services Sector
Table 34: Global Market for CDx Services, by Region, Through 2028
Table 35: Global Market for CDx Services, by Application, Through 2028
Table 36: Global Market for Companion Diagnostics, by Application, Through 2028
Table 37: Global Market for Companion Diagnostics in Cancer Applications, by Region, Through 2028
Table 38: Cancer Incidence, Global, by Cancer Type, 2022
Table 39: Approved CDx Drugs for Cancer Treatment, by Cancer Type, 2023
Table 40: Global Market for Companion Diagnostics in Cancer Applications, by Cancer Type, Through 2028
Table 41: Global Market for Companion Diagnostics in Neurologic Disorder Applications, by Region, Through 2028
Table 42: Global Market for Companion Diagnostics in Neurologic Disorder Applications, by Type, Through 2028
Table 43: Global Market for Companion Diagnostics in Cardiovascular Disease Applications, by Region, Through 2028
Table 44: Global Market for Companion Diagnostics in Infectious Disease Applications, by Region, Through 2028
Table 45: Global Market for Companion Diagnostics in Other Disease Applications, by Region, Through 2028
Table 46: Global Market for Companion Diagnostics, by End User, Through 2028
Table 47: Global Market for Companion Diagnostics in Clinical Laboratories, by Region, Through 2028
Table 48: Recent Collaborations and Partnerships in the Companion Diagnostics Market, 2022 and 2023
Table 49: Global Market for Companion Diagnostics in Pharmaceutical Companies, by Region, Through 2028
Table 50: Global Market for Companion Diagnostics in Contract Research Organizations, by Region, Through 2028
Table 51: Global Market for Companion Diagnostics in Other End Users, by Region, Through 2028
Table 52: Global Market for Companion Diagnostics, by Region, Through 2028
Table 53: North American Market for Companion Diagnostics, by Country, Through 2028
Table 54: European Market for Companion Diagnostics, by Country, Through 2028
Table 55: Asia-Pacific Market for Companion Diagnostics, by Country, Through 2028
Table 56: FDA Approved CDx, by Company, as of December 2023
Table 57: Companion Diagnostics: Market Share Analysis, 2023
Table 58: Leading Companies in the Companion Diagnostics Market, by Technology
Table 59: Companion Diagnostics: Market Ranking, by Major Technology
Table 60: Grants and Funding in Companion Diagnostics, 2020-2022
Table 61: Recent Developments in the Companion Diagnostics Market, 2023
Table 62: Clinical Trials in Companion Diagnostics, by Type of Study, January 2024
Table 63: Clinical Trials in Companion Diagnostics, by Status, January 2024
Table 64: Clinical Trials in Companion Diagnostics, by Phase, January 2024
Table 65: Clinical Trials in Companion Diagnostics, by Region, January 2024
Table 66: Patents Granted on Companion Diagnostics, by Top Applicant, 2019-2023
Table 67: Patents Granted on Companion Diagnostics, by Top Owner, 2019-2023
Table 68: Patents Granted for Companion Diagnostics, by Jurisdiction, 2019-2023
Table 69: ESG Risk Rankings for Companion Diagnostic Companies, 2024*
Table 70: ESG: Environmental Overview
Table 71: ESG: Social Overview
Table 72: ESG: Governance Overview
Table 73: Acronyms Used in This Report
Table 74: Abbott: Company Snapshot
Table 75: Abbott: Financial Performance, FY 2022 and 2023
Table 76: Abbott: Product Portfolio
Table 77: Agilent Technologies Inc.: Company Snapshot
Table 78: Agilent Technologies Inc.: Financial Performance, FY 2022 and 2023
Table 79: Agilent Technologies Inc.: Product Portfolio
Table 80: Agilent Technologies Inc.: News/Key Developments, 2021-2023
Table 81: ARUP Laboratories: Company Snapshot
Table 82: ARUP Laboratories: Product Portfolio
Table 83: ARUP Laboratories: News/Key Developments, 2023
Table 84: bioMérieux: Company Snapshot
Table 85: bioMérieux: Financial Performance, FY 2021 and 2022
Table 86: bioMérieux: Product Portfolio
Table 87: bioMérieux: News/Key Developments, 2021
Table 88: Danaher Corp.: Company Snapshot
Table 89: Danaher Corp.: Financial Performance, FY 2022 and 2023
Table 90: Danaher Corp.: Product Portfolio
Table 91: Danaher Corp.: News/Key Developments, 2021-2023
Table 92: F. Hoffmann-La Roche Ltd.: Company Snapshot
Table 93: F. Hoffmann-La Roche Ltd.: Financial Performance, FY 2021 and 2022
Table 94: F. Hoffmann-La Roche Ltd.: Product Portfolio
Table 95: F. Hoffmann-La Roche Ltd.: News/Key Developments, 2021-2023
Table 96: Illumina Inc.: Company Snapshot
Table 97: Illumina Inc.: Financial Performance, FY 2022 and 2023
Table 98: Illumina Inc.: Product Portfolio
Table 99: Illumina Inc.: News/Key Developments, 2021 and 2022
Table 100: Myriad Genetics Inc.: Company Snapshot
Table 101: Myriad Genetics Inc.: Financial Performance, FY 2022 and 2023
Table 102: Myriad Genetics Inc.: Product Portfolio
Table 103: Myriad Genetics Inc.: News/Key Developments, 2020 and 2023
Table 104: QIAGEN: Company Snapshot
Table 105: QIAGEN: Financial Performance, FY 2021 and 2022
Table 106: QIAGEN: Product Portfolio
Table 107: QIAGEN: News/Key Developments, 2021-2023
Table 108: Thermo Fisher Scientific Inc.: Company Snapshot
Table 109: Thermo Fisher Scientific Inc.: Financial Performance, FY 2022 and 2023
Table 110: Thermo Fisher Scientific Inc.: Product Portfolio
Table 111: Thermo Fisher Scientific Inc.: News/Key Developments, 2020-2023
List of Figures
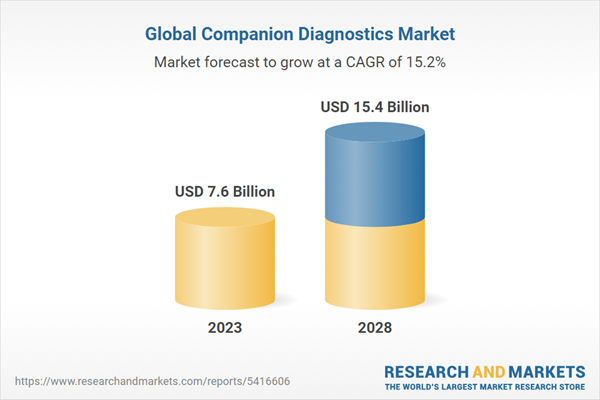
Summary Figure A: Global Market for Companion Diagnostics, by Product, 2020-2028
Summary Figure B: Global Market for Companion Diagnostics, by Region, 2020-2028
Figure 1: Companion Diagnostics: Timeline, 1990s-Present
Figure 2: Timeline of FDA Guidance Release for Companion Diagnostics (CDx), as of 2023
Figure 3: Market Dynamics of Companion Diagnostics
Figure 4: Share of Global Cancer Incidence for Both Sexes, by Region, 2022
Figure 5: Companion Diagnostics: Growing Share in Total New Molecular Entities (NMEs), 2019-2022
Figure 6: Emerging Trends/Technologies in the Companion Diagnostics Market
Figure 7: Patents Granted for Companion Diagnostics, by Year, 2019-2023
Figure 8: ESG Pillars
Figure 9: Abbott: Revenue Share, by Business Unit, FY 2023
Figure 10: Abbott: Revenue Share, by Country/Region, FY 2023
Figure 11: Agilent: Revenue Share, by Business Unit, FY 2023
Figure 12: Agilent: Revenue Share, by Country/Region, FY 2023
Figure 13: bioMérieux: Market Share, by Business Unit, FY 2022
Figure 14: bioMérieux: Revenue Share, by Country/Region, FY 2022
Figure 15: Danaher Corp.: Market Share, by Business Unit, FY 2023
Figure 16: Danaher Corp.: Revenue Share, by Country/Region, FY 2023
Figure 17: F. Hoffmann-La Roche Ltd.: Revenue Share, by Business Unit, FY 2022
Figure 18: F. Hoffmann-La Roche Ltd.: Revenue Share, by Country/Region, FY 2022
Figure 19: Illumina Inc.: Revenue Share, by Business Unit, FY 2022
Figure 20: Illumina Inc.: Revenue Shares, by Country/Region, FY 2022
Figure 21: Myriad Genetics Inc.: Revenue Share, by Business Unit, FY 2023
Figure 22: Myriad Genetics Inc.: Revenue Share, by Country/Region, FY 2023
Figure 23: QIAGEN: Market Share, by Business Unit, FY 2022
Figure 24: QIAGEN: Revenue Share, by Country/Region, FY 2022
Figure 25: Thermo Fisher Scientific Inc.: Market Share, by Business Unit, FY 2023
Figure 26: Thermo Fisher Scientific Inc.: Revenue Share, by Country/Region, FY 2023