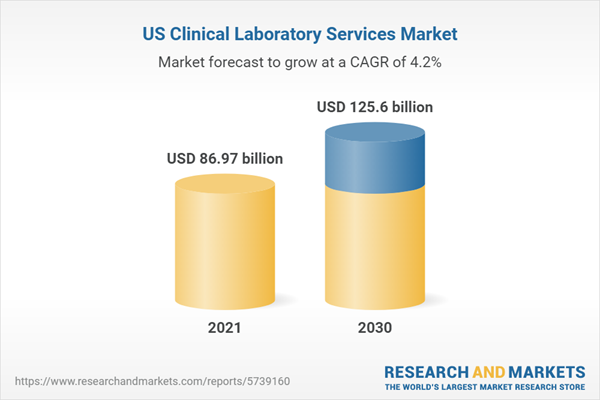
The US clinical laboratory services market is worth approximately $86.97 billion and is projected to expand to $125.6 billion by 2030. The market is growing at a CAGR of 4.2%.US Clinical Laboratory Services Market to Surpass Valuation of 125.6 billion by 2030
The demand for US clinical laboratory services market is growing rapidly. This is due to the increasing number of people suffering from diseases and disorders that require diagnosis and treatment. Clinical laboratories play a vital role in the healthcare system, providing essential information that helps doctors and nurses to make informed decisions about patient care.
With the advent of new technologies, clinical laboratories are now able to offer a wider range of services than ever before. This means that they are able to meet the needs of a growing number of patients. However, this also means that they are under pressure to keep up with the latest developments in order to remain competitive in the US market.
As the demand for clinical laboratory services market continues to grow, so too does the need for qualified staff. There is a shortage of trained professionals who are able to work in these settings in the US, which is putting strain on the existing workforce. This is likely to lead to higher wages for those with the necessary skills and experience.
Market Dynamics
Drivers
- An aging population: As people live longer, they require more medical care and testing. This is especially true of Older Americans, who tend to have more chronic conditions that require regular monitoring
- The growth of preventive medicine: There is an increasing emphasis on preventing disease rather than simply treating it. This has led to more testing in order to catch problems early
- The rise of personalized medicine: Advances in technology have allowed for tests that are tailored to an individual's unique genetic makeup. This has led to a need for more specialized lab services
- A growing awareness of health risks: With the advent of the Internet and social media, people are increasingly aware of the importance of maintaining their health. This has resulted in more demand for clinical lab services as people seek ways to monitor their health status
- The declining reimbursement rates are a major challenge for clinical laboratories in the US. The Centers for Medicare and Medicaid Services (CMS) have been reducing the reimbursement rates for clinical laboratory services over the past few years. This has led to a decrease in the revenue of clinical laboratories, which has, in turn, hampered their ability to invest in new technology and infrastructure
- The lack of standardized regulations is another major challenge for clinical laboratories in the US. Unlike many other countries, there is no central body that regulates all aspects of the clinical laboratory industry in the US. This has led to a situation where each state has its own set of rules and regulations, which can vary significantly from one another. This can make it very difficult for clinical laboratories to operate across multiple states or even just stay compliant with all the different rules and regulations
Segmental Overview
By Type
By test type, clinical chemistry testing is projected to offers a growth opportunity of over $9,000 million in the US clinical laboratory services market during the forecast period, 2022-2030.Clinical chemistry testing is a vital and growing area of diagnostic medicine. The demand for these services is increasing in the United States, as more and more healthcare providers recognize the value of these tests in identifying and treating disease.
Clinical chemistry tests are used to measure a variety of substances in the body, including electrolytes, enzymes, proteins, hormones, and other chemicals. These tests can provide important information about a patient's overall health status and can help guide treatment decisions.
The field of clinical chemistry is constantly evolving, as new technologies and approaches are developed to improve the accuracy and precision of these tests. This evolution will continue to drive the demand for these services in the years to come.
On the other hand, immunology testing to grow at the highest CAGR of 5.7% in the US clinical laboratory services market.
By Application
By application, the bioanalytical & lab chemistry services held over 60% share of the US clinical laboratory services market in the year 2021 and the segment is projected to continue to keep its dominance intact in the forthcoming years. However, toxicology testing services to grow at the highest CAGR of 5.2%.There is currently a high demand for bioanalytical and lab chemistry services. This is due to the increasing popularity of biochemical and pharmaceutical research, as well as the need for more accurate and precise data in these fields.
Bioanalytical services are used to study the structure and function of biomolecules, including proteins, DNA, and enzymes. They are also used to measure the levels of metabolites in cells and tissues. Lab chemistry services are used to synthesize chemicals, determine their purity, and analyze their properties.
Both bioanalytical and lab chemistry services are essential for advances in biomedical research. Without these services, it would be difficult to obtain the detailed data needed to understand the complex processes involved in human health and disease.
When it comes to developing new products, time is of the essence. That's why many companies are turning to bioanalytical and lab chemistry services to help them get a jump on the competition. By outsourced these services, companies can save precious time and resources that can be better spent elsewhere.
By End Users
Hospital laboratories are the largest consumers in the US clinical laboratory services market. The report, titled 'The Future of Hospital Laboratories,' found that hospitals account for nearly 45% of all spending on clinical laboratory services in the US, which is also expected to generate revenue of over 50,000 million by the end of 2028.The report's authors attribute this high level of consumption to the growing complexity of hospital care and the increasing use of diagnostic tests and procedures. They also note that hospitals are under pressure to cut costs, which has led to a shift in spending from in-patient to out-patient services.
Despite these challenges, the authors believe that hospitals can remain a key driver of growth in the clinical laboratory industry by investing in new technologies and consolidating their operations.
Top Players in the US clinical Laboratory Services Market
The cumulative market share of the top fifteen major players is close to 25%- Qiagen Inc
- Opko Health, Inc
- Abbott Laboratories
- Charles River Laboratories
- Johnson & Johnson
- Roche Laboratories
- Astrazeneca
- Arup Laboratories
- Davita, Inc
- Pfizer Inc
- Eli Lilly
- Novartis Laboratories
- Merck Inc
- Siemens Healthcare Limited
- Viapath Group Llp
- Almac Group
- Neogenomics Laborateries
- Other Prominent Players
Segmental Analysis
By Test Type
- Clinical Chemistry Testing
- Endocrinology Chemistry Testing
- Routine Chemistry Testing
- Therapeutic Drug Monitoring (TDM) Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Genetic Testing
- Drug of Abuse Testing
By Applications
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Others
By End User
- Hospitals
- Government Agencies
- Physicians
- Clinicals Labs
- Pharmaceutical Companies
- Others
U.S. Clinical Laboratory Services Market Report Answers Questions Such As:
- What is the market size and forecast of the U.S Clinical Laboratory Services Market?
- What are the inhibiting factors and impact of COVID-19 on the U.S Clinical Laboratory Services Market during the assessment period?
- Which are the products/segments/applications/areas to invest in over the assessment period in the U.S Clinical Laboratory Services Market?
- What is the competitive strategic window for opportunities in the U.S Clinical Laboratory Services Market?
- What are the technology trends and regulatory frameworks in the U.S Clinical Laboratory Services Market?
- What is the market share of the leading players in the U.S Clinical Laboratory Services Market?
- What modes and strategic moves are considered favorable for entering the U.S Clinical Laboratory Services Market?
Value Addition:
- Technology Outlook in the Clinical Laboratory Industry
Table of Contents
Chapter 1. Research Framework1.1 Research Objective
1.2 Product Overview
1.3 Market Segmentation
Chapter 2. Research Methodology
2.1 Qualitative Research
2.1.1 Primary & Secondary Sources
2.2 Quantitative Research
2.2.1 Primary & Secondary Sources
2.3 Breakdown of Primary Research Respondents, By Region
2.4 Assumption for the Study
2.5 Market Size Estimation
2.6. Data Triangulation
Chapter 3. Executive Summary: USA Clinical Laboratory Services (Reference labs) Market
Chapter 4. USA Clinical Laboratory Services (Reference labs) Market Overview
4.1. Industry Value Chain Analysis
4.1.1. Material Provider
4.1.2. Service Provider
4.1.3. End User
4.2. Industry Outlook
4.2.1. Technology Outlook in the Clinical Laboratory Industry
4.2.2. Reference la bs - Current status in U.S.
4.3. PESTLE Analysis
4.4. Porter's Five Forces Analysis
4.4.1. Bargaining Power of Suppliers
4.4.2. Bargaining Power of Buyers
4.4.3. Threat of Substitutes
4.4.4. Threat of New Entrants
4.4.5. Degree of Competition
4.5. Market Dynamics and Trends
4.5.1. Growth Drivers
4.5.2. Restraints
4.5.3. Challenges
4.5.4. Key Trends
4.6. Covid-19 Impact Assessment on Market Growth Trend
4.7. Market Growth and Outlook
4.7.1. Market Revenue Estimates and Forecast (US$ Mn), 2017 - 2030
4.7.3. Price Trend Analysis, By Product
4.8. Competition Dashboard
4.8.1. Market Concentration Rate
4.8.2. Company Market Share Analysis (Value %), 2020
4.8.3. Competitor Mapping
Chapter 5. Clinical Laboratory Services (Reference labs) Market Analysis, By Test Type
5.1. Key Insights
5.2. Market Size and Forecast, 2017 - 2030 (US$ Mn)
5.2.1. Clinical Chemistry Testing
5.2.1.1. Routine Chemistry Testing
5.2.1.2. Endocrinology Chemistry Testing
5.2.1.3. Therapeutic Drug Monitoring (TDM) Testing
5.2.1.4. Specialized Chemistry Testing
5.2.1.5. Other Clinical Chemistry Testing
5.2.2. Microbiology Testing
5.2.2.1. Infectious Disease Testing
5.2.2.2. Transplant Diagnostic Testing
5.2.2.3. Other Microbiology Testing
5.2.3. Hematology Testing
5.2.4. Immunology Testing
5.2.5. Cytology Testing
5.2.6. Genetic Testing
5.2.7. Drug of Abuse Testing
Chapter 6. Clinical Laboratory Services (Reference labs) Market Analysis, By Application
6.1. Key Insights
6.2. Market Size and Forecast, 2017 - 2030 (US$ Mn)
6.2.1. Bioanalytical & Lab Chemistry Services
6.2.2. Toxicology Testing Services
6.2.3. Cell & Gene Therapy Related Services
6.2.4. Preclinical & Clinical Trial Related Services
6.2.5. Drug Discovery & Development Related Services
6.2.6. Others
Chapter 7. Clinical Laboratory Services (Reference labs) Market Analysis, By End Users
7.1. Key Insights
7.2. Market Size and Forecast, 2017 - 2030 (US$ Mn)
7.2.1. Physicians
7.2.2. Government Agencies
7.2.3. Hospitals
7.2.4. Clinical Labs
7.2.5. Pharmaceutical Companies
7.2.6. Others
Chapter 8.
- Company Profile (Company Overview, Financial Matrix, Key Product landscape, Key Personnel, Key Competitors, Contact Address, and Business Strategy Outlook)
8.2. Opko Health, Inc.
8.3. Abbott Laboratories
8.4. Charles River Laboratories
8.5. Johnson & Johnson
8.6. Roche Laboratories
8.7. Pfizer Inc
8.8. Eli Lilly
8.9. Novartis Laboratories
8.10. Merck Inc.
8.11. Astrazeneca
8.12. Arup Laboratories
8.13. Davita, Inc.
8.14. Siemens Healthcare Limited
8.15. Viapath Group Llp
8.16. Almac Group
8.17. Neogenomics Laborateries
8.18. Eurofins Scientific
8.19. UNILABS, SYNLAB International GmbH
8.20. H.U. Groups Holdings, Inc.
8.21. Sonic Healthcare
8.22. ACM Global Laboratories
8.23. Amedes Holding GmbH
8.24. BioReference Laboratories, Inc.
Executive Summary
The US clinical laboratory services market is worth approximately $86.97 billion and is projected to expand to 125.6 billion by 2030. The market is growing at a CAGR of 4.2%, driven by advances in medical technology and the increasing demand for diagnostic testing.There are more than 7,000 clinical laboratories in the US, ranging from large national chains to small independent operations. The top 15 laboratories account for around 15% of market revenue. The US clinical laboratory services market is highly fragmented, with no single player accounting for more than 3% of revenue. Some of the largest laboratories are Quest Diagnostics, LabCorp, and Sonic Healthcare.
Consolidation is occurring within the clinical laboratory services industry, as larger players look to acquire smaller rivals to gain market share. In recent years, there have been a number of high-profile mergers and acquisitions.
One key trend is the increasing use of point-of-care testing (POCT). This type of testing is typically done outside of traditional laboratory settings and can offer quicker results for patients. POCT also offers opportunities for labs to expand their reach into new markets and patients.
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Qiagen Inc.
- Opko Health, Inc.
- Abbott Laboratories
- Charles River Laboratories
- Johnson & Johnson
- Roche Laboratories
- Astrazeneca
- Arup Laboratories
- Davita, Inc.
- Pfizer Inc
- Eli Lilly
- Novartis Laboratories
- Merck Inc.
- Siemens Healthcare Limited
- Viapath Group Llp
- Almac Group
- Neogenomics Laborateries
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 124 |
| Published | December 2022 |
| Forecast Period | 2021 - 2030 |
| Estimated Market Value ( USD | $ 86.97 billion |
| Forecasted Market Value ( USD | $ 125.6 Billion |
| Compound Annual Growth Rate | 4.2% |
| Regions Covered | United States |