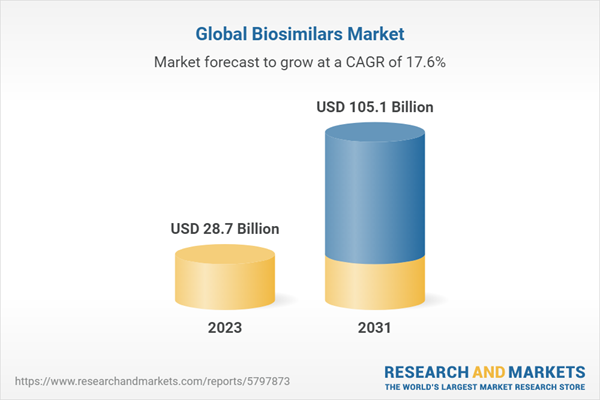
The biosimilars market size attained a value of USD 24.5 billion in 2022. The market is likely to grow at a CAGR of 17.60% during the forecast period of 2023-2031, likely to attain a value of USD 105.1 billion by 2031.
Biosimilars Market: Introduction and Application
Biosimilars are biological products that are highly similar to an already approved and marketed reference biologic drug, with no clinically meaningful differences in terms of safety, purity, and potency. They are derived from living organisms, such as bacteria, yeast, or mammalian cells, and are used to treat various diseases and medical conditions. Biosimilars are developed after the patent expiration of the original biologic drug, also known as the innovator product.
Biosimilars are used to treat a wide range of conditions, including autoimmune disorders (examples- rheumatoid arthritis and inflammatory bowel disease), cancer, diabetes, and blood disorders (examples- anaemia and neutropenia). They are used as therapeutic proteins, monoclonal antibodies, and hormones, among other applications.
The benefits of biosimilars include the following:
- Cost Savings: As biosimilars are often less expensive than the reference biologic drugs, they can lead to substantial cost savings for healthcare systems and patients. This affordability increases patient access to essential medicines and treatments
- Competition: The introduction of biosimilars fosters market competition, which can drive down the prices of biologic drugs and improve their availability
- Innovation: Developing biosimilars requires extensive research and development, which can lead to the discovery of new therapeutic strategies and enhancements in biopharmaceutical manufacturing processes
- Expanding Treatment Options: The availability of biosimilars can provide healthcare professionals and patients with additional treatment options, leading to more personalized and effective care
Biosimilars Market Segmentations
The market can be categorised into product type, drug type, indication, procedure, and region.
Market by Product Type
- Recombinant Glycosylated Proteins
- Monoclonal Antibodies
- Erythropoietin
- Recombinant Non-Glycosylated Proteins
- Insulins
- Granulocyte Colony Stimulating Factors
- Interferons
- Others
Market Breakup by Drug Class
- Insulin
- Recombinant Human Growth Hormone (RHGH)
- Granulocyte Colony-Stimulating Factor
- Interferon
- Erythropoietin
- Etanercept
- Monoclonal Antibodies
- Follitropin
- Glucagon
- Calcitonin
- Teriparatide
- Enoxaparin Sodium
- Others
Market Breakup by Indications
- Chronic Diseases
- Oncology
- Autoimmune Diseases
- Infectious Diseases
- Blood Disorders
- Growth Hormone Deficiency
- Others
Market Breakup by Procedure
- Invasive
- Non-Invasive
Biosimilars Market Breakup by Region
North America
- United States of America
- Canada
Europe
- United Kingdom
- Germany
- France
- Italy
- Others
Asia Pacific
- China
- Japan
- India
- ASEAN
- Australia
- Others
Latin America
- Brazil
- Argentina
- Mexico
- Others
Middle East and Africa
- Saudi Arabia
- United Arab Emirates
- Nigeria
- South Africa
- Others
Biosimilars Market Scenario
The global biosimilars market has been witnessing rapid growth, driven by factors such as patent expiration of blockbuster biologic drugs, increasing prevalence of chronic diseases, and rising demand for cost-effective treatment options. Regulatory support and streamlined approval pathways have facilitated the entry of biosimilars into the market, leading to increased competition and more affordable healthcare options.
In addition, the growing awareness of biosimilars among healthcare professionals and patients has further fuelled market expansion. Emerging markets, such as those in Asia Pacific and Latin America, offer significant growth potential due to their large patient populations and improving healthcare infrastructure. Key industry players are focusing on strategic partnerships, collaborations, and mergers and acquisitions to strengthen their product pipelines, expand their global presence, and capitalize on the growing market opportunities. Overall, the biosimilars market scenario presents a promising outlook, with the potential to significantly improve patient access to life-changing therapies and alleviate the financial burden on healthcare systems worldwide.
Key Players in the Global Biosimilars Market
The report gives an in-depth analysis of the key players involved in the biosimilars market, sponsors manufacturing the drugs, and putting them through trials to get FDA approvals. The companies included in the market are as follows:
- Novartis AG
- Orion Pharma
- Pfizer Inc
- Samsung Bioepis
- Coherus BioSciences, Inc
- Amgen Inc
- Eli Lilly and Company
- Takeda Pharmaceutical Company Limited
- Bristol-Myers Squibb Company
- Merck KGaA
- Teva Pharmaceutical Industries Ltd
- Biocon
- Bayer AG
- AbbVie Inc,
- Allergan
- Dr. Reddy’s Laboratories Ltd
- Boehringer Ingelheim International GmbH
- Biogen
Table of Contents
Companies Mentioned
- Novartis AG
- Orion Pharma
- Pfizer Inc.
- Samsung Bioepis
- Coherus Biosciences, Inc.
- Amgen Inc.
- Eli Lilly and Company.
- Takeda Pharmaceutical Company Limited.
- Bristol-Myers Squibb Company
- Merck Kgaa
- Teva Pharmaceutical Industries Ltd.
- Biocon
- Bayer AG
- Abbvie Inc,
- Allergan
- Dr. Reddy’S Laboratories Ltd.
- Boehringer Ingelheim International Gmbh.
- Biogen
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 147 |
| Published | April 2023 |
| Forecast Period | 2023 - 2031 |
| Estimated Market Value ( USD | $ 28.7 Billion |
| Forecasted Market Value ( USD | $ 105.1 Billion |
| Compound Annual Growth Rate | 17.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 18 |