Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
These include a rising demand for painless and convenient vaccination methods, increased focus on preventive healthcare, and the development of innovative nasal vaccine formulations. Nasal vaccines are particularly appealing for children and individuals who fear needles, making them a preferred choice for immunization. The market has seen the expansion of the types of vaccines delivered nasally.
Initially, nasal vaccines were primarily associated with influenza vaccines, but now they cover a wider range of diseases, including COVID-19, measles, mumps, rubella, and more. This diversification has significantly boosted the market. Pharmaceutical companies and research institutions are actively investing in R&D to develop more effective and potent nasal vaccine formulations. This includes enhancing the stability and delivery mechanisms of nasal vaccines.
Governments and international organizations are increasingly recognizing the potential of nasal vaccines in improving vaccination coverage rates. This has led to greater investments in research and vaccination campaigns using nasal vaccines, particularly in low-income and middle-income countries. Public awareness campaigns and educational efforts are playing a vital role in increasing the acceptance of nasal vaccines. These campaigns highlight the advantages of nasal vaccines, such as reduced pain, easier administration, and quicker immunity development.
Ky Market Drivers
Growing Prevalence of Infectious and Respiratory Diseases
The rising incidence of infectious and respiratory diseases is a key driver fueling the expansion of the global nasal vaccines market. As the global burden of diseases such as influenza, COVID-19, tuberculosis (TB), and respiratory syncytial virus (RSV) continues to escalate, there is an increasing demand for effective, accessible, and non-invasive vaccination solutions. The global healthcare sector faces significant challenges due to the widespread prevalence of seasonal influenza and tuberculosis (TB). Each year, seasonal influenza affects approximately one billion people worldwide, with 3 to 5 million cases progressing to severe illness. The disease is responsible for an estimated 290,000 to 650,000 respiratory-related deaths annually, creating substantial strain on healthcare systems and economic productivity.Similarly, tuberculosis (TB) remains a critical global health challenge. In 2023 alone, an estimated 10.8 million people contracted TB worldwide, affecting 6.0 million men, 3.6 million women, and 1.3 million children. The disease continues to impact all countries and age groups, reinforcing its widespread public health burden. Despite being both curable and preventable, TB persists due to gaps in early detection, treatment access, and healthcare infrastructure. Nasal vaccines provide a targeted immune response, particularly against airborne pathogens, making them a highly attractive alternative to traditional injectable vaccines. Respiratory diseases are among the leading causes of morbidity and mortality worldwide.
Influenza alone affects millions annually, leading to hospitalizations, economic strain, and loss of productivity. Similarly, emerging infectious diseases like COVID-19 have reinforced the need for scalable and easily deployable vaccination strategies. In 2020, chronic obstructive pulmonary disease (COPD) affected an estimated 10.6% of the global population, translating to 480 million cases across both men and women.
This chronic respiratory condition continues to expand as a major public health concern, with projections indicating a 23.3% increase in cases by 2050. By that time, the total number of COPD cases is expected to reach 592 million, representing 9.5% of the global at-risk population. Nasal vaccines are particularly effective in preventing respiratory diseases because they mimic the natural infection route of airborne viruses and bacteria. Countries with high infection rates are increasingly investing in preventive immunization strategies, thereby accelerating the adoption of nasal vaccine technologies.
Key Market Challenges
Regulatory Hurdles
One of the primary challenges facing the nasal vaccines market is navigating the complex regulatory landscape. The approval process for nasal vaccines can be more challenging compared to traditional injectable vaccines. Regulators need to ensure the safety and efficacy of these vaccines, which can lead to longer development timelines and higher costs. To address this challenge, collaboration between vaccine developers and regulatory agencies is essential to streamline the approval process and establish clear guidelines for nasal vaccine development.Key Market Trends
Technological Advancements
The global healthcare landscape is constantly evolving, with technological advancements at the forefront of innovation. One significant development is the growing interest in nasal vaccines, a novel approach to immunization that offers numerous advantages over traditional injection-based vaccines. These advancements have ignited a surge in the Global Nasal Vaccines Market, shaping the future of preventive medicine and public health.Advancements in vaccine formulation have played a pivotal role in the growth of nasal vaccines. Researchers are developing new formulations that enhance vaccine stability, improve antigen delivery, and minimize side effects, thereby increasing their efficacy and safety. Adjuvants are substances added to vaccines to boost the body's immune response. Recent developments in adjuvant technology have led to the creation of nasal vaccines that can trigger stronger and more durable immune responses, improving their overall effectiveness.
Key Market Players
- Vaxart, Inc.
- FluGen Inc.
- Altimmune, Inc.
- Sinovac Biotech Ltd.
- Sanofi Pasteur SA
- Pfizer Inc.
- GlaxoSmithKline plc
- Johnson & Johnson
- Ennaid Therapeutics, LLC
Report Scope:
In this report, the Global Nasal Vaccines Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Nasal Vaccines Market, By Vaccine Type:
- Live Attenuated Vaccines
- Inactivated Vaccines
- Subunit
- Recombinant and Conjugate Vaccines
- Others
Nasal Vaccines Market, By Application:
- Influenza
- COVID-19
- Others
Nasal Vaccines Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Nasal Vaccines Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Vaxart, Inc.
- FluGen Inc.
- Altimmune, Inc.
- Sinovac Biotech Ltd.
- Sanofi Pasteur SA
- Pfizer Inc.
- GlaxoSmithKline plc
- Johnson & Johnson
- Ennaid Therapeutics, LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | March 2025 |
| Forecast Period | 2024 - 2030 |
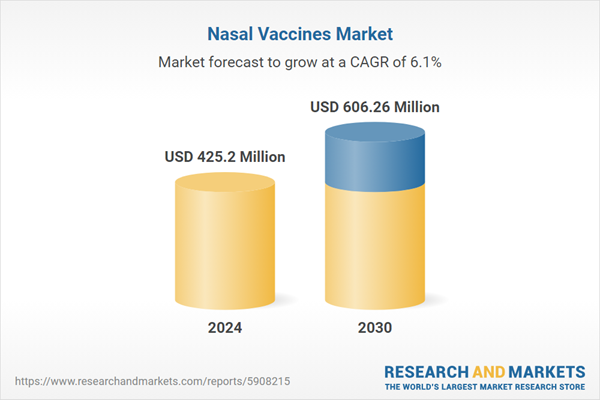
| Estimated Market Value ( USD | $ 425.2 Million |
| Forecasted Market Value ( USD | $ 606.26 Million |
| Compound Annual Growth Rate | 6.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 9 |