Sezary Syndrome: Introduction
Sezary syndrome is a rare and fast-growing skin and blood cancer. It is a cutaneous T-cell lymphoma that affects the skin, bloodstream, and lymph nodes. It may induce a change in skin color, making it appear red or dark and causing itchy and painful rashes. The early symptoms may look like eczema or psoriasis but during the later stages, the skin develops small bumps. Blood tests, biopsies, CT scans and PET scans are commonly used to diagnose the condition.Sezary Syndrome Market Analysis
Sezary syndrome is a type of cutaneous T-cell lymphoma which may occur with mycosis fungoides. There is no ultimate cure for the condition, however, early detection of the condition can help patients live symptom free life for years. Consequently, the Sezary syndrome market growth is driven by the rising healthcare advancements to develop better health diagnostic methods, with a specific emphasis on depleting the number of cancer related mortality rates.Sezary syndrome is often treated with topical therapies, systemic therapies, or both. Topical or skin directed are generally advised for early stages of the disease. Topical corticosteroids and topical chlormethine may be used on their own or in combination with systemic options for advanced stages. Bexarotene gel, an FDA approved topical retinoid, is also used as topical treatment. Apart from topical therapies, ultraviolet phototherapy, photodynamic therapy, total skin electron beam therapy and localized radiotherapies are used to eradicate the tumor from the body.
The Sezary syndrome market demand is driven by the rising advancements and applications of systemic therapies to treat the condition. With systemic therapies, the response rates and durations are comparatively shorter. Targeted immunotherapies, especially monoclonal antibodies, are gaining high preference when it comes to treating tumors. Currently, drug combinations like Mogamulizumab + LD TSEBT are under clinical trials and have completed the phase 2 stage.
Sezary Syndrome Market Segmentation
“Sezary Syndrome Market Report and Forecast 2025-2034” offers a detailed analysis of the market based on the following segments:Market Breakup by Treatment Type
- Standard Treatment
- Advance Treatment
Market Breakup by Diagnosis
- Immunophenotyping
- T-Cell Receptor
- Gene Rearrangement Test
- Others
Market Breakup by Drug Class
- Monoclonal Antibody
- Retinoid
- Histone Deacetylase Inhibitor
- Biologic Response Modifier
- Antibody-Drug Conjugate
- Corticosteroids
- Immune Stimulants
Market Breakup by Route of Administration
- Oral
- Parenteral
Market Breakup by Region
- United States
- United Kingdom
- Germany
- France
- Italy
- Spain
- Japan
Sezary Syndrome Market Overview
The United States has held a major Sezary syndrome market share in the historical period. In June 2023, Kyowa Kirin, Inc., launched ‘Proactively Recognizing Occurrence in Blood through Education (PROBE)' initiative to educate the healthcare professionals on the importance of weighing the blood involvement in Sezary syndrome. Such initiatives demonstrate the rising emphasis on early detection of the condition to prevent future adversities.Europe is another major market for Sezary syndrome, with a robust healthcare system that aims at offering quality treatment to the patients. Having well-equipped research labs and clinics, the region is a major leader in offering innovative drugs and therapies. The market is also affected by the increasing number of drug approvals and collaborations between research institutions and pharmaceutical companies.
The Asia Pacific region, especially countries like Japan, are enhancing their healthcare infrastructure to address the Sezary syndrome market demand. Several companies are expanding their product portfolios to cater to growing need of new drugs and topicals for treating the condition. Asia Pacific is also the center for foreign investments with prominent companies setting up research labs in the region to leverage the easy availability of resources.
Sezary Syndrome Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Kyowa Kirin Co.
- Gilead Sciences
- Seattle Genetics
- Merck
- Amerigen Pharmaceuticals Limited
- STI Pharma
- Bioniz Therapeutics
- Minophagen Pharmaceutical Co.
- Bayer AG
- Novartis AG
- Shionogi Inc.
- Eisai Co.
- Hikma Pharmaceuticals PLC
- Innate Pharma
- BE Biopharma
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Kyowa Kirin Co.
- Gilead Sciences
- Seattle Genetics
- Merck
- Amerigen Pharmaceuticals Limited
- STI Pharma
- Bioniz Therapeutics
- Minophagen Pharmaceutical Co.
- Bayer AG
- Novartis AG
- Shionogi Inc.
- Eisai Co.
- Hikma Pharmaceuticals PLC
- Innate Pharma
- BE Biopharma
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
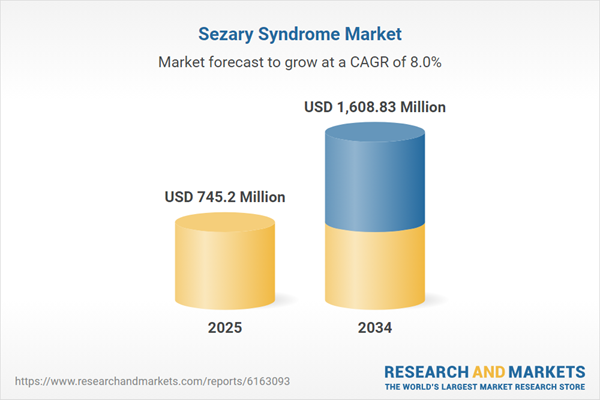
| Estimated Market Value ( USD | $ 745.2 Million |
| Forecasted Market Value ( USD | $ 1608.83 Million |
| Compound Annual Growth Rate | 8.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |