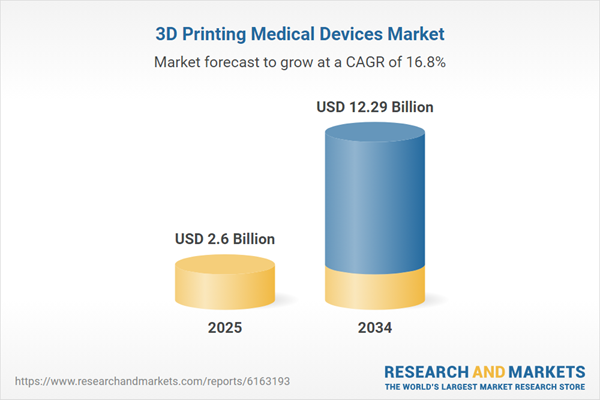
Global 3D Printing Medical Devices Market Overview
The growth of the market can be attributed to the increased adoption of 3D printing technologies in fields such as of dental laboratories, and hearing aid manufacturers. Consequently, the market is expected to witness a significant increase in demand for 3D printed medical devices.The global 3D printing medical devices market growth is driven by continuous ongoing innovations and the increasing interest of key players in investing in this potential opportunity to fill a crucial gap in the market. The 3D printed medical devices market is witnessing substantial competition as most technology companies are interested in expanding their portfolio by stepping into the healthcare industry. This competition is enabling more tech companies to increase interest in adopting this technology from hospitals and surgical centres across the globe. The increased adoption of 3D printing is expected to create several opportunities for investors, looking to capitalize in the healthcare sector. Further, the increasing number of innovative start-ups in the 3D printing ecosystem is collectively contributing to the rising 3D printing medical devices market share.
Strategic Collaborations to Drive the Market Growth
The market growth can be attributed to the increasing collaboration efforts, such as the joint venture between Ulsan National Institute of Science and Technology and Pusan National University Yangsan Hospital, showcasing innovation and the development of advanced 3D printing medical device technologies. Such collaboration activities highlight the market's shift towards collaborative research and commercialization, contributing to a conducive environment for transformative medical solutions. Additionally, the market is equally driven by the increasing research initiatives like the Polina project, supported by the European Innovation Council. These research activities showcase the researcher's commitment to making use of bioprinting to create safe, cost-effective medical devices with structures imitating natural tissues. Such innovative research activities may be a major catalyst in further driving the global 3D printing medical devices market growth in the forecast period.Further, In December 2023, Singapore General Hospital (SGH) is collaborating with Nanyang Technological University, Singapore (NTU Singapore) to set up a Joint Research & Development Laboratory in additive manufacturing (AM), also known as 3D printing. This collaboration is expected to benefit SGH patients from healthcare innovations, such as customized medical devices and implants that are 3D printed. These innovations are currently under development and are expected to represent a significant leap toward innovative healthcare solutions that could redefine patient treatment. This collaboration will make use of the combined expertise and resources of SGH's 3D Printing Centre and NTU's Singapore Centre for 3D Printing (SC3DP) to further study and develop related technologies for clinical applications in a point-of-care setup.
Increasing Investments to Aid the Market Development
Furthermore, the market growth is also influenced by substantial funding activities such as Zeda's successful series B financing round. Zeda is a California-based nanotech and 3D manufacturing company that has raised a whopping amount of USD 52 million. Such fundraising activities may act as catalysts in the market expansion as with funds now available Zeda can expand across the geographical boundaries, driving the global 3D printing medical devices market demand.Research and Development to Support the Market Growth
The market is expected to witness advanced research activities targeted toward bringing innovative 3D printed solutions for medical situations such as 3D printed organs mimicking real organs. For instance, according to a post on MIT News in February 2023, MIT engineers are developing customized treatments for patients' specific heart forms and functions, with a custom robotic heart. The team has allegedly developed a procedure to 3D print a soft and flexible replica of a patient's heart. The replica can then be controlled to mimic that patient's blood-pumping ability. The procedure involves first converting medical images of a patient's heart into a three-dimensional computer model, which the researchers can then 3D prints using a polymer-based ink. The result is a soft, flexible shell in the exact shape of the patient's own heart. The team can also use this approach to print a patient's aorta - the major artery that carries blood out of the heart to the rest of the body.Global 3D Printing Medical Devices Market Segmentations
3D Printing Medical Devices Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Product Type
- Equipment
- Materials
- Services and Software
Market Breakup by Technology
- Laser Beam Melting
- Photopolymerization
- Droplet Deposition/Extrusion-based Technologies
- Electron Beam Melting
- Three-dimensional Printing/Adhesion Bonding/Binder Jetting
- Other Technologies
Market Breakup by Applications
- Surgical Guides
- Surgical Instruments
- Standard Prosthetics and Implants
- Custom Prosthetics and Implants
- Tissue-Engineered Products
- Hearing Aids
- Wearable Medical Devices
- Other Applications
Market Breakup by End User
- Hospitals and Surgical Centers
- Dental and Orthopedic Clinics
- Academic Institutions and Research Laboratories
- Pharma-Biotech and Medical Device Companies
- Clinical Research Organizations
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global 3D Printing Medical Devices Market Regional Analysis
North America is expected to dominate the global market and is expected to dominate throughout the forecast period owing to factors like the comprehension of advanced technologies and their widespread applications in different areas. The medical devices industry in North America is popular due to its high-quality product offerings with the use of advanced technology resulting from significant investment in research and development. The reliance on the quality aspect is a major factor in the growth of the market.The market is poised for significant growth in the forecast period owing to factors such as the increasing interest in developing innovative solutions, exemplified by Stratasys and CollPlant's recent collaboration. Their idea for regenerative breast implants is an innovative approach to tissue and organ bioprinting. It is expected to bring a paradigm shift in breast cancer treatment, modifying immune responses and facilitating natural tissue regeneration. These innovative ideas and approaches are a major avenue in showcasing rising innovation and the potential of this advanced technique in the forecast period, expected to bolster the global 3D printing medical devices market growth.
Global 3D Printing Medical Devices Market: Competitor Landscape
In June 2023, EOS partnered with Tecomet, Inc., Precision ADM, and Orthopaedic Innovation Centre (OIC) to offer a medical device-enabling solution. While Precision ADM is a well-known Canadian engineering and 3D printing firm with deep experience in making titanium medical devices, OIC is a testing company for medical devices.The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:
- Stratasys Ltd.
- EnvisionTEC
- Koninklijke Philips N.V.
- 3D Systems, Inc.
- EOS
- Renishaw plc.
- Materialise
- 3T Additive Manufacturing Ltd.
- GENERAL ELECTRIC COMPANY
- Carbon, Inc.
- Prodways Group
- SLM Solutions
- Organovo Holdings Inc.
- Anatomics Pty Ltd
- Groupe Gorge
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Stratasys Ltd.
- EnvisionTEC
- Koninklijke Philips N.V.
- 3D Systems, Inc.
- EOS
- Renishaw plc.
- Materialise
- 3T Additive Manufacturing Ltd.
- GENERAL ELECTRIC COMPANY
- Carbon, Inc.
- Prodways Group
- SLM Solutions
- Organovo Holdings Inc.
- Anatomics Pty Ltd.
- Groupe Gorgé
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 2.6 Billion |
| Forecasted Market Value ( USD | $ 12.29 Billion |
| Compound Annual Growth Rate | 16.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |