Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Also, with the shift towards personalized medicine and targeted therapies in oncology, precise tumor characterization facilitated by core needle biopsies is increasingly crucial. Initiatives such as public awareness campaigns and organized breast cancer screening programs encourage women to undergo routine screenings, resulting in higher detection rates and an increased demand for biopsy procedures.
Key Market Drivers
Technological Innovations
Advancements in imaging technologies such as ultrasound, mammography, and MRI have significantly improved the accuracy of image-guided core needle biopsies. Real-time imaging enables precise guidance of the biopsy needle to the target area, enhancing tissue sampling accuracy. Digital breast tomosynthesis, or 3D mammography, offers clearer and more detailed breast tissue images, aiding in biopsy accuracy through multiple views from different angles. Robotic-assisted biopsy systems provide heightened precision and control, minimizing variability from human factors and improving sampling accuracy. Vacuum-assisted biopsy (VAB) systems use vacuum pressure to gently draw tissue into the biopsy needle, enhancing efficiency and diagnostic yield. Non-invasive localization systems, enabled by wireless technology, assist surgeons in precisely locating and removing marked breast lesions post-biopsy. Integrated software tools with imaging systems aid in planning biopsy paths, calculating needle trajectory, and optimizing sample collection, resulting in more accurate and efficient biopsies. Dual-Energy Contrast-Enhanced Digital Mammography combines contrast-enhanced mammography with 3D imaging, facilitating better visualization of cancer-associated abnormal blood vessels and guiding more accurate biopsies.Specialized biopsy devices designed for MRI-guided biopsies are crafted from non-magnetic materials, ensuring compatibility with MRI scanners. Liquid biopsies, though not traditional core needle biopsies, involve analyzing blood samples for circulating tumor DNA (ctDNA) or biomarkers, offering genetic tumor insights without tissue sampling. Artificial Intelligence (AI) and Machine Learning enhance medical image analysis, aiding in identifying subtle abnormalities and assisting in biopsy path planning and lesion nature prediction. Needle design innovations prioritize reduced tissue damage, improved accuracy, and patient comfort. Features like self-contained spring-loaded needles and beveled-tip designs, equipped with sensors for real-time feedback on tissue stiffness and proximity to critical structures, elevate sampling precision. These advancements collectively contribute to the growth of the Global Breast Cancer Core Needle Biopsy Market.
Growing Geriatric Population
As the population ages, the occurrence of various health conditions, including breast cancer, tends to rise. Breast cancer risk escalates with age, particularly among the elderly population, necessitating more frequent screenings and diagnostic procedures like core needle biopsies. Older individuals face a heightened risk of delayed diagnosis, often stemming from factors such as reduced participation in screening programs. Such delays may lead to the requirement for more invasive diagnostic measures like core needle biopsies. Elderly patients commonly present with comorbidities and complex medical backgrounds, complicating treatment decisions. Core needle biopsies offer crucial insights into tumor characteristics, facilitating appropriate treatment planning. Tailored treatment plans, considering factors like overall health, medical history, and cancer aggressiveness, are often necessary for older patients, and core needle biopsies play a pivotal role in providing the requisite information.Core needle biopsies are generally less invasive with shorter recovery periods compared to surgical biopsies, a significant advantage for older patients with potentially diminished physical resilience. Breast cancer screening guidelines advocate regular mammograms for specific age groups of women. As women age, the likelihood of requiring further evaluation via core needle biopsies due to suspicious mammogram findings rises. With advancements in healthcare and quality of life, many seniors are living longer, making timely detection and diagnosis of breast cancer increasingly critical for maintaining quality of life. Early detection significantly enhances treatment outcomes, especially considering the heightened risk of aggressive tumors among the geriatric population. As the demand for geriatric healthcare services surges, facilities specializing in elderly care are more inclined to provide comprehensive diagnostic services, including core needle biopsies. Public health campaigns targeting breast cancer awareness among the elderly encourage participation in screenings and diagnostic procedures, further fueling demand for the Global Breast Cancer Core Needle Biopsy Market.
Screening and Awareness Campaigns
These campaigns are designed to educate the public about breast cancer detection, promote regular screenings, and advocate for early diagnosis. By highlighting the importance of early detection in improving breast cancer outcomes, they stimulate demand for diagnostic procedures like core needle biopsies. Encouraging individuals, especially those at higher risk or in specific age groups, to undergo breast cancer screenings leads to increased detection of suspicious lesions requiring further evaluation through core needle biopsies. Routine screenings, such as mammograms, often reveal abnormalities requiring additional testing, potentially resulting in core needle biopsy recommendations to determine the nature of the abnormality.Awareness campaigns play a vital role in educating individuals about the diagnostic process, including core needle biopsies, to alleviate concerns and promote acceptance of the procedure. Targeting specific demographics, such as particular age groups or high-risk populations, ensures that those who would benefit most from core needle biopsies understand their importance. Promoting routine health check-ups, during which healthcare providers may recommend screenings and subsequent diagnostic procedures like core needle biopsies, is a key aspect of these campaigns.
Empowering individuals with knowledge about their healthcare options encourages proactive behavior, such as requesting necessary diagnostic procedures. Successful awareness campaigns lead to more early-stage breast cancer diagnoses, potentially reducing breast cancer mortality rates. Community events, workshops, and support groups organized as part of these campaigns create a sense of community and encourage individuals to prioritize their health, including undergoing necessary diagnostic procedures. This dynamic contributes to the increasing demand in the Global Breast Cancer Core Needle Biopsy Market.
Key Market Challenges
Cost and Reimbursement Issues
These challenges can impact both healthcare providers and patients, affecting the adoption and accessibility of core needle biopsy procedures. Healthcare facilities need to invest in specialized equipment, imaging technologies, and trained personnel to offer core needle biopsies. The initial investment required for these resources can be substantial. Core needle biopsies involve various costs, including equipment maintenance, consumables, imaging guidance, and pathology analysis. These costs can contribute to the overall expenses of the procedure. Healthcare providers often rely on reimbursements from insurance companies or government programs to cover the costs of medical procedures. Inadequate reimbursement rates for core needle biopsies might discourage providers from offering these services. Reimbursement policies can vary based on factors such as the specific procedure, facility type, geographical region, and insurance coverage. This variability can lead to uncertainty for both providers and patients. In some cases, insurance plans might not cover the full cost of core needle biopsies or might not cover them at all. This financial burden can discourage patients from undergoing the procedure. Healthcare providers often need to go through pre-authorization processes, submitting detailed documentation to insurance companies to demonstrate the medical necessity of the procedure. This administrative burden can delay patient care. Patients may be required to cover a portion of the procedure's cost through co-pays, deductibles, or other out-of-pocket expenses. High out-of-pocket costs might deter patients from choosing core needle biopsies.Diagnostic Accuracy and False Positives/Negatives
Accurate diagnostic results from core needle biopsies are essential for determining the nature of breast lesions. Oncologists rely on these results to formulate effective treatment plans tailored to each patient's condition. False positives (incorrectly identifying cancer) and false negatives (missing cancer) can lead to inappropriate or delayed treatment decisions. Accurate biopsy results are essential for choosing the most suitable treatment strategy. False-positive results can lead to unnecessary anxiety and stress for patients, while false negatives might lead to delayed diagnosis and treatment, negatively affecting patient outcomes and mental well-being. In cases of inconclusive or inaccurate core needle biopsy results, patients might need to undergo rebiopsy or additional diagnostic procedures, increasing healthcare costs and patient discomfort. Incorrect diagnoses can strain healthcare resources by requiring additional medical interventions and treatments that could have been avoided with accurate initial diagnoses. Healthcare providers need to implement quality control and assurance measures to minimize the risk of false results. Ensuring consistent accuracy across different facilities and professionals can be challenging. Accurate interpretation of core needle biopsy samples requires skilled pathologists who can differentiate between various breast tissue abnormalities. Ensuring enough skilled pathologists is essential.Key Market Trends
Preference for Minimally Invasive Procedures
Minimally invasive procedures involve smaller incisions or needle insertions, leading to less tissue trauma and reduced post-procedure discomfort and pain. Patients are more likely to choose core needle biopsies over surgical options due to the associated comfort. Minimally invasive procedures typically result in quicker recovery times compared to traditional surgeries. This is especially beneficial for patients who wish to return to their daily activities as soon as possible. Many minimally invasive core needle biopsies can be performed on an outpatient basis. This convenience allows patients to undergo the procedure and return home on the same day, reducing the need for hospitalization. Minimally invasive techniques usually carry a lower risk of infection, bleeding, and other complications commonly associated with more invasive surgical procedures. Minimally invasive procedures often leave smaller scars or no visible scars, which can be important for patients concerned about cosmetic outcomes. Core needle biopsies are often performed under local anesthesia, reducing the risks associated with general anesthesia and allowing patients to remain awake and comfortable during the procedure. The preference for minimally invasive procedures aligns with patients' desire for less invasive approaches to healthcare. Patients are more likely to agree to recommended procedures when they are perceived as less invasive. Minimally invasive core needle biopsies can be performed in a variety of medical settings, including clinics and outpatient centers, making them more accessible to a broader patient population.Segmental Insights
Technology Insights
In 2023, the Global Breast Cancer Core Needle Biopsy Market dominated by MRI-Based Breast Biopsy segment in the forecast period and is predicted to continue expanding over the coming years. MRI (Magnetic Resonance Imaging) offers high-resolution images of breast tissue, making it highly accurate in identifying suspicious lesions or abnormalities. This accuracy is crucial for guiding the biopsy needle to the precise location of the abnormality, which enhances the diagnostic yield of the biopsy. MRI is particularly effective in detecting and characterizing complex breast lesions that might be difficult to visualize using other imaging modalities. This makes it a valuable tool for identifying lesions that require further investigation through biopsy. For individuals with a high risk of breast cancer due to family history or genetic mutations (e.g., BRCA1/BRCA2), MRI-based screening and biopsies can offer better sensitivity in detecting early-stage cancers or precancerous lesions.Regional Insights
The North America region dominates the Global Breast Cancer Core Needle Biopsy Market in 2023. North America, particularly the United States and Canada, has a well-developed and advanced healthcare infrastructure. This includes state-of-the-art medical facilities, research institutions, and a robust regulatory environment that fosters innovation and rapid adoption of new medical technologies. The region has a history of early adoption of advanced medical technologies. This includes diagnostic tools and procedures like breast cancer core needle biopsies. Healthcare providers in North America are often quick to embrace new techniques that can enhance patient care and outcomes. Many leading medical device manufacturers and biotechnology companies are based in North America. This concentration of research and development efforts contributes to the creation of innovative biopsy devices and techniques that attract global attention. North America places a significant emphasis on cancer screening, early detection, and diagnosis. This focus drives the demand for accurate and minimally invasive diagnostic procedures like core needle biopsies.Report Scope:
In this report, the Global Breast Cancer Core Needle Biopsy Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:
Breast Cancer Core Needle Biopsy Market, By Technology:
- MRI-Based Breast Biopsy
- Ultrasound-Based Breast Biopsy
- Mammography-Based (Stereotactic) Breast Biopsy
- CT-Based Breast Biopsy
- Other Image Based Breast Biopsy
Breast Cancer Core Needle Biopsy Market, By End-user:
- Hospitals & Diagnostic Laboratories
- Pharmaceutical & Biotechnology companies
- Academic & Research Institutes
Breast Cancer Core Needle Biopsy Market, By region:
- North America
- United States
- Canada
- Mexico
- Asia-Pacific
- China
- India
- South Korea
- Australia
- Japan
- Europe
- Germany
- France
- United Kingdom
- Spain
- Italy
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Breast Cancer Core Needle Biopsy Market.Available Customizations:
Global Breast Cancer Core Needle Biopsy Market report with the given market data, the publisher offers customizations according to a company's specific needs.This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Intact Medical Corporation
- Ethicon Endo Surgery
- Galini SRL
- Leica Biosystems Nussloch GmbH
- Hologic Inc.
- Argon Medical Devices
- Encapsule Medical Devices LLC.
- Cook Medical Incorporated
- Becton & Dickinson Company
- C.R. Bard, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | May 2024 |
| Forecast Period | 2024 - 2029 |
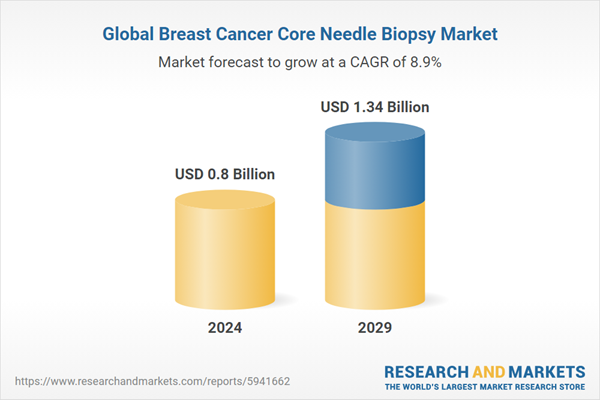
| Estimated Market Value ( USD | $ 0.8 Billion |
| Forecasted Market Value ( USD | $ 1.34 Billion |
| Compound Annual Growth Rate | 8.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |