Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
The growing prevalence of diseases, such as cancer and neurodegenerative disorders, has intensified research efforts to develop new therapies and treatments. Cell viability assays play a critical role in understanding disease mechanisms and testing potential interventions. Ongoing advancements in life sciences research, including fields like cancer research, stem cell research, and regenerative medicine, require reliable methods to assess cell viability and behavior under different conditions. The shift towards personalized medicine involves tailoring treatments to individual patient characteristics. Cell viability assays are essential in evaluating how a patient's cells respond to specific treatments, aiding in the development of personalized therapeutic approaches. Events like the COVID-19 pandemic have underscored the importance of rapid and accurate viability assays for studying infectious diseases, testing antiviral compounds, and evaluating vaccine candidates. Continuous technological innovations in assay platforms, imaging techniques, and detection methods are enhancing the sensitivity, precision, and speed of cell viability assays, making them more versatile and valuable.
Key Market Drivers
Rise in Drug Discovery and Development
In the early stages of drug discovery, researchers screen numerous compounds to pinpoint potential drug candidates. Cell viability assays swiftly evaluate their impact on cell health, aiding in candidate selection. Once promising candidates emerge, these assays gauge their effectiveness in targeting specific disease pathways or cellular processes. They offer insights into differential viability effects on diseased versus healthy cells. Ensuring drug safety is paramount, with cell viability assays used to assess compound toxicity, detecting adverse effects before advancing to further studies.Determining the dose-response relationship of a drug candidate is crucial for understanding the optimal dosage for therapeutic effect while minimizing toxicity. Viability assays provide information on how a drug candidate affects cellular pathways and processes, aiding in understanding its mechanism of action and therapeutic potential. Personalized medicine requires evaluating individual patient responses to different drug candidates, with viability assays guiding effective treatment options.
Viability assays also validate potential drug targets, helping researchers understand their relevance in disease pathways. The demand for high-throughput screening has led to the development of automated assays, increasing drug discovery efficiency. As biologics and cell-based therapies advance, viability assays play a vital role in assessing cell health and functionality. They also identify potential interactions between drugs, supporting the development of combination therapies, crucial for the Global Cell Viability Assays Market.
Emerging Infectious Diseases and Vaccines
These assays are pivotal in comprehending the impact of infectious agents, assessing potential antiviral compounds, and evaluating vaccine candidates. During outbreaks of emerging infectious diseases like COVID-19, there is an urgent need for effective antiviral drugs. Cell viability assays are crucial for screening compounds to identify those inhibiting viral replication while safeguarding host cells. They aid in understanding antiviral compounds' mechanisms by assessing their effects on infected cells, crucial for optimizing drug candidates and developing targeted therapies. These assays also facilitate the assessment of existing drugs for potential antiviral activity against new infectious agents, expediting drug development using known compounds with established safety profiles.Also, they are integral in evaluating vaccine efficacy, assessing immune response stimulation, and measuring the ability to prevent infection or reduce viral replication. Adjuvants, which enhance vaccine immune-stimulating effects, are also evaluated for safety and effectiveness using cell viability assays. Determining vaccine immunogenicity, the ability to induce an immune response, is vital for predicting vaccine effectiveness and finding the balance between efficacy and safety. These assays are also employed to assess vaccine stability under various storage conditions, ensuring effectiveness during distribution.
Researchers utilize cell viability assays to study virus effects on cellular health, aiding in understanding infection mechanisms and intervention development. High-throughput screening methods, facilitated by automated assays, are essential for addressing urgent infectious diseases. As new infectious agents emerge, reliable methods such as cell viability assays become crucial for research, thereby driving demand in the Global Cell Viability Assays Market.
Technological Advancements
These advancements have broadened researchers' capabilities in evaluating cell health, screening compounds, and studying diseases more effectively. Label-free assays, like impedance-based assays, track changes in electrical impedance as cells attach, proliferate, or perish. They offer real-time monitoring without external labels, enabling dynamic and non-invasive viability assessment. High-Content Screening (HCS) integrates automated microscopy and image analysis to evaluate multiple cellular parameters concurrently, providing deeper insights into cell behavior. Fluorescent probes and dyes have been developed to target specific cellular components associated with viability, enhancing assay specificity and enabling multiplexing for comprehensive data.Nanotechnology-based biosensors detect cellular changes at the nanoscale, enabling highly sensitive and specific viability measurements by identifying subtle alterations in cellular physiology. With the rising popularity of 3D cell culture models, viability assays have adapted to assess cell health in these complex environments using techniques like confocal imaging and specialized viability markers.
Microfluidic devices and organ-on-a-chip platforms replicate physiological conditions, offering more realistic viability assessments. They shed light on how cells respond to drugs and toxins in environments resembling the human body. Live-cell imaging, such as time-lapse microscopy, tracks changes in cell viability over time, providing insights into cell health dynamics and treatment responses. Automation has transformed cell viability assays, enhancing throughput and reducing errors through robotic platforms and digital microscopy with advanced image analysis software.
Advancements in single-cell analysis unveil viability heterogeneity within populations, uncovering rare subpopulations with distinct behaviors. Microscale Thermophoresis (MST) measures changes in the movement of fluorescently labeled molecules in response to temperature changes, offering insights into viability-related pathways by assessing binding interactions. Molecular assays, like real-time polymerase chain reaction (PCR), gauge changes in gene expression linked to cell viability, apoptosis, and stress responses. These advancements will accelerate the demand for the Global Cell Viability Assays Market.
Key Market Challenges
Variability in Assay Performance
Different cell lines, even of the same type, can exhibit varying sensitivities to assays due to genetic differences, culture conditions, and intrinsic characteristics. Within a cell population, individual cells can respond differently to treatments or conditions. This heterogeneity can introduce variability in viability measurements. Variations in assay procedures, such as incubation times, reagent concentrations, and detection methods, can lead to inconsistent results between experiments or laboratories. Manual steps in assays can introduce variability due to differences in technique, timing, and subjective judgment. Differences in instruments, such as microplate readers or imaging systems, can lead to variations in measurements, affecting assay consistency. Environmental conditions like temperature and humidity can influence cell behavior and assay outcomes. Variations in these conditions can lead to inconsistent results. Primary cells, cell lines, and patient-derived samples can behave differently in assays, leading to variability. Variability can arise during sample collection, processing, and preparation, affecting assay outcomes.Sensitivity and Specificity
Achieving the right balance between sensitivity (the ability to detect small changes) and specificity (the ability to accurately identify true positives) is essential for the reliability and relevance of viability assay results. An overly sensitive assay might detect even minor changes in cell behavior that are not biologically relevant. This can lead to false positives and misinterpretation of results. Viability assays should be sensitive enough to detect subtle changes in cell health caused by treatments or conditions, especially in cases where the impact is not immediately obvious. Cells can exhibit dynamic responses to stimuli, including transient changes in viability. Ensuring that an assay can capture these dynamic shifts requires careful design and optimization. Cells within a population can exhibit inherent variability in response to external factors. A sensitive assay should account for this natural variability without generating excessive noise. Lack of specificity can result in false-negative results, where the assay fails to identify actual viability changes. This can lead to missing critical biological responses. Different cell types can respond differently to the same conditions. An assay might be specific in one cell type but not in another, affecting its generalizability. Components of the assay, such as dyes or substrates, can interact with cells or compounds in unintended ways, leading to nonspecific signals.Key Market Trends
Environmental Testing
Cell viability assays are employed to assess the potential toxic effects of pollutants and chemicals on various organisms present in the environment, including aquatic species and plants. These assays help evaluate the impact of contaminants on the health and survival of these organisms. Environmental testing is not limited to ecological impacts; it also involves assessing the effects of pollutants on human health. Cell viability assays can be used to study how environmental factors influence human cells and tissues, aiding in understanding exposure-related health risks. Environmental contaminants known as endocrine disruptors can interfere with hormone systems and have adverse effects on both wildlife and humans. Cell viability assays are used to study the effects of these disruptors on cellular health and hormone pathways. Cell viability assays are adaptable to assess air and water quality. For example, airborne particulates or waterborne pollutants can be tested using assays designed to measure cellular responses to contaminants. Agricultural chemicals, such as pesticides and herbicides, can enter the environment and affect non-target organisms. Cell viability assays assist in evaluating the toxicity of these chemicals to both target and non-target species. Residues from pharmaceuticals and personal care products can end up in the environment, potentially affecting aquatic ecosystems. Cell viability assays help assess the impact of these residues on aquatic organisms.Segmental Insights
Product Insights
In 2023, the Global Cell Viability Assays Market dominated by consumables segment in the forecast period and is predicted to continue expanding over the coming years. The usefulness of consumables in numerous pharmaceutical and biopharmaceutical, diagnostic, and stem cell research applications can be credited with this. Additionally, several market participants are providing a large selection of non-toxic, ready-to-use, and high-quality results-immediate cell viability assay reagents.Luminometric assays are anticipated to witness the fastest growth in the forecast period. Because of their straightforward equipment requirements, straightforward methods, resilience, and exceptional sensitivity. Due to its advantages over colorimetric and dye exclusion assays because they are more sensitive, fluorometric assays held the second-largest share in 2023. All these characteristics make it simple to scale and adapt from bench research to high throughput applications. Such characteristics of the cell viability assay are promoting the growth of the consumables segment.
End-user Insights
In 2023, the Global Cell Viability Assays Market biopharmaceutical and pharmaceutical companies segment held the largest share and is predicted to continue expanding over the coming years. Since viability assays are frequently used in the pharmaceutical industry to assess how developed drugs affect cells. To track the effectiveness of therapies that have been created and frequently target cancer tumours, researchers employ a wide variety of assays. Additionally, a cell viability assay can be used to gauge the compounds' or agents' degrees of toxicity. The segment is driven by the biopharmaceutical and pharmaceutical industries' numerous applications of cell viability assay.CROs and CMOs are expected to grow at the fastest growth in the forecast period. For a variety of reasons, pharmaceutical companies collaborate with CROs and CMOs. These partnerships may save costs, speed up time to market, and enhance process effectiveness. Collaborations are anticipated to expand the use of cell viability assays across a range of applications, including drug discovery and development, supporting industry growth over the next few years.
Regional Insights
The North America region dominates the Global Breast Cancer Core Needle Biopsy Market in 2023. This significant increase in investment initiatives by the government, the rise in the prevalence of chronic diseases like cancer, and the presence of a top-notch infrastructure for clinical and laboratory research in North America are all factors that contribute to this significant share. For instance, according to Globocanreport, there were almost 612,390 fatalities and 2,281,658 new cancer cases in the U.S. in 2020. As a result, the market's growth possibilities are expanding due to the rising incidence of chronic and infectious diseases as well as the growing emphasis on cell-based treatments.Asia Pacific is predicted to experience the fastest growth during the projection period. Due to the increasing need for innovative therapies in the region. Other elements promoting regional prosperity include escalating government expenditure in R&D and quickening infrastructure growth. Additionally, funding is being provided to several local players to work on creating a cure for a number of chronic illnesses, including cancer.
Report Scope:
In this report, the Global Cell Viability Assays Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Cell Viability Assays Market, By Product:
- Consumables
- Reagents and Assay Kits, by type
Colorimetric assays
Fluorometric assays
Luminometric assays
- Instruments
- Spectrophotometer
- Microscopy
- Cell imaging and analysis system
- Flow Cytometry
- Others
Cell Viability Assays Market, By Application:
- Drug Discovery and Development
- Stem Cell Research
- Diagnostics
Cell Viability Assays Market, By End User:
- Biopharmaceutical & Pharmaceutical Companies
- CROs & CMOs
- Academic & Research Institutes
- Diagnostic Labs
Global Cell Viability Assays Market, By region:
- North America
- United States
- Canada
- Mexico
- Asia-Pacific
- China
- India
- South Korea
- Australia
- Japan
- Europe
- Germany
- France
- United Kingdom
- Spain
- Italy
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Cell Viability Assays Market.Available Customizations:
Global Cell Viability Assays Market report with the given market data, the publisher offers customizations according to a company's specific needs.This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Thermo Fisher Scientific, Inc.
- Agilent Technologies, Inc.
- Bio-Rad Laboratories, Inc.
- Merck KGaA
- PerkinElmer Inc.
- Promega Corporation
- Biotium Inc.
- Creative Bioarra
- Abcam plc
- Charles River Laboratories International, Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | May 2024 |
| Forecast Period | 2024 - 2029 |
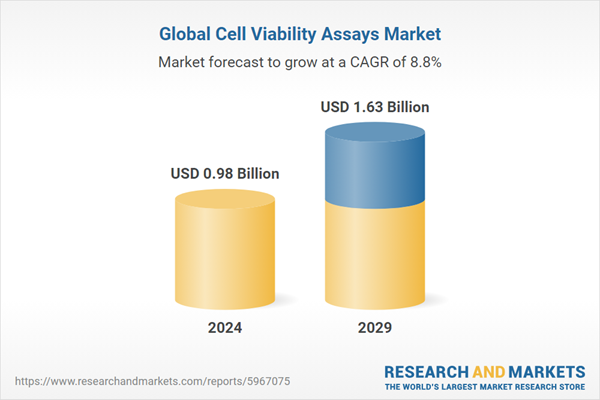
| Estimated Market Value ( USD | $ 0.98 Billion |
| Forecasted Market Value ( USD | $ 1.63 Billion |
| Compound Annual Growth Rate | 8.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |