The frontotemporal dementia market has been comprehensively analyzed in this report titled "Frontotemporal Dementia Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". Frontotemporal dementia is an early-onset neurodegenerative disorder caused by progressive neuron damage in the frontal and temporal lobes of the brain. Depending on the initial affected area in the brain, the condition is classified into behavioral-variant frontotemporal dementia, primary progressive aphasia, semantic dementia, and progressive non-fluent aphasia. The symptoms of frontotemporal dementia worsen over time and differ from person to person. The common indications include significant changes in personal and social behavior, blunting of emotions, apathy, deficits in both receptive and expressive language, etc. Individuals suffering from frontotemporal dementia may also experience uncontrolled eating, lack of self-control, difficulty speaking or understanding speech, trouble communicating, loss of reading and writing skills, etc. The diagnosis involves an evaluation of a family history of frontotemporal dementia, the patient's clinical presentation, and a physical exam. Various imaging studies, including MRI, CT scan, PET scan, etc., are used to detect the affected parts of the brain. The healthcare professional may also perform a lumbar puncture to confirm a diagnosis and rule out other health conditions.
The rising cases of genetic mutations, which affect the production of proteins in the brain, leading to cell death and brain damage, are primarily driving the frontotemporal dementia market. In addition to this, the escalating utilization of neuropsychological testing for assessing changes in cognitive function and identifying specific patterns of impairment consistent with the disease is also propelling the global market. Furthermore, the widespread adoption of antidepressants and antipsychotics to cope with various behavioral problems among patients suffering from frontotemporal dementia is creating a positive outlook for the market. Besides this, the emerging popularity of non-pharmacological interventions, such as behavioral, physical, occupational, and speech therapy, which prevent disruptive behaviors and provide symptom relief, is also bolstering the market growth. Additionally, numerous key players are making extensive investments in R&D activities to launch innovative treatment procedures, including gene therapy, to potentially reduce or cease disease progression. This, in turn, is acting as another significant growth-inducing factor. Moreover, the introduction of favorable criteria to enable early approvals of pipeline medications for neurodegenerative illnesses and clinical studies that encourage the entry of new products is further expected to drive the frontotemporal dementia market in the coming years.
This report provides an exhaustive analysis of the frontotemporal dementia market in the United States, EU4 (Germany, Spain, Italy, and France), United Kingdom, and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report, the United States has the largest patient pool for frontotemporal dementia and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario, unmet medical needs, etc., have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the frontotemporal dementia market in any manner.
Recent Developments:
In February 2024, Neuroscientists at Macquarie University in Australia developed a single-dose genetic therapy that has been proven to stop the progression of both ALS and frontotemporal dementia in mice. This new treatment, called CTx1000, targets abnormal buildups of the protein TDP-43 in cells in the spinal cord and brain, which has been connected with frontotemporal dementia.
In January 2024, Coya Therapeutics, Inc. disclosed that it is expanding its pipeline in neurological disorders for COYA 302, including frontotemporal dementia. COYA 302 is a dual-mechanism investigational biologic combination immunotherapy, which has the potential to modify ailment by targeting multiple dysregulated immune pathways while restoring activity in anti-inflammatory Treg function.
In December 2023, Passage Bio, Inc. announced preliminary safety and biomarker data from three Cohort 1 participants in the ongoing global Phase 1/2 upliFT-D clinical trial examining PBFT02, an adeno-associated virus (AAV)-delivery genetic therapy for the treatment of frontotemporal dementia with granulin (GRN) mutations.
In July 2023, Arkuda Therapeutics presented pre-clinical results on its lead development candidate, ARKD-104, for the treatment of GRN-related frontotemporal dementia at the 2023 Alzheimer's Association International Conference (Poster P1-752). The data presented reveal that ARKD-104, a novel, brain-penetrant, orally available small molecule, boosts PGRN in the central nervous system of non-human primates in a dose-dependent manner. Furthermore, ARKD-104 was found to enhance key cofactors of lysosomal enzymes involved in brain activity.
Key Highlights:
Frontotemporal dementia is the second most frequent form of dementia in people younger than 65 years, and its incidence is predicted to increase as the population ages.
Researchers estimate that around 55 million people worldwide have dementia. Of that number, 10-20% have frontotemporal dementia.
In the United States, researchers estimated that the prevalence of frontotemporal dementia among adults aged 45 to 65 years ranged between 15 to 22 per 100,000, with incidence estimates ranging from 2.7 to 4.1 per 100,000 individuals in the same age range.
This condition can last from 2 to 20 years, with an average duration of 8 years from the onset of symptoms.
A higher frequency of females has been demonstrated among frontotemporal dementia patients harboring GRN mutation.
Drugs:
DNL593 is a replacement treatment for frontotemporal dementia resulting from granulin gene abnormalities. The therapeutic substance is made up of progranulin protein combined with an antibody fragment that bonds with the transferrin receptor. This association with transferrin receptors on BBB endothelial cells enhances the receptor-mediated transcytosis of progranulin protein into the neural system.
PR-006 is under clinical development by Prevail Therapeutics for the management of frontotemporal dementia with GRN mutation. This gene therapy is administered intracisternally into the cerebrospinal fluid and involves non-replicating recombinant adeno-associated virus serotype 9 to transmit codon-optimized DNA expressing wild-type progranulin.
PBFT02 is an investigational gene therapy to treat frontotemporal dementia. It uses an AAV1 viral vector to deliver a functional GRN gene to patients with mutations in the progranulin gene (PGRN) via ICM. This vector and delivery strategy aims to supply higher-than-normal amounts of the PGRN protein to the CNS, addressing progranulin insufficiency in GRN gene mutation carriers.
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the frontotemporal dementia market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the frontotemporal dementia market
Competitive Landscape:
This report also provides a detailed analysis of the current frontotemporal dementia marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the frontotemporal dementia market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the frontotemporal dementia market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the frontotemporal dementia market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of frontotemporal dementia across the seven major markets?
- What is the number of prevalent cases (2018-2034) of frontotemporal dementia by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of frontotemporal dementia by gender across the seven major markets?
- How many patients are diagnosed (2018-2034) with frontotemporal dementia across the seven major markets?
- What is the size of the frontotemporal dementia patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of frontotemporal dementia?
- What will be the growth rate of patients across the seven major markets?
Frontotemporal Dementia: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for frontotemporal dementia drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the frontotemporal dementia market?
- What are the key regulatory events related to the frontotemporal dementia market?
- What is the structure of clinical trial landscape by status related to the frontotemporal dementia market?
- What is the structure of clinical trial landscape by phase related to the frontotemporal dementia market?
- What is the structure of clinical trial landscape by route of administration related to the frontotemporal dementia market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 138 |
| Published | May 2024 |
| Forecast Period | 2023 - 2034 |
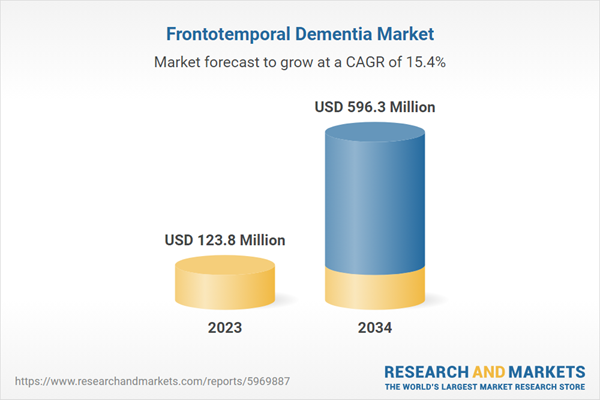
| Estimated Market Value ( USD | $ 123.8 Million |
| Forecasted Market Value ( USD | $ 596.3 Million |
| Compound Annual Growth Rate | 15.4% |
| Regions Covered | Global |