Global Orphan Drugs Market - Key Trends & Drivers Summarized
Why Are Orphan Drugs Becoming Increasingly Important in Healthcare?
Orphan drugs are becoming a significant focus in the pharmaceutical industry, driven largely by the need to address rare diseases that affect a small percentage of the population but have historically been neglected due to limited market potential. These drugs are specifically developed to treat, prevent, or diagnose rare medical conditions, often called orphan or rare diseases, which affect fewer than 200,000 individuals per year in the United States. The growing awareness of rare diseases, coupled with advocacy efforts from patient groups and non-profit organizations, has put pressure on pharmaceutical companies to focus on developing treatments for these underserved conditions. Government incentives, such as market exclusivity, tax credits, and accelerated approvals, have played a crucial role in encouraging pharmaceutical companies to invest in orphan drug development.How Is Innovation Shaping the Orphan Drugs Market?
Technological and scientific advancements have significantly boosted the development of orphan drugs, particularly through advances in genomics, personalized medicine, and biotechnology. Innovations such as CRISPR gene editing, targeted therapies, and monoclonal antibodies have revolutionized the ability to understand the underlying genetic and molecular causes of rare diseases, making it possible to create more effective and targeted treatments. Additionally, the increasing use of biomarkers for precise diagnosis and drug matching is paving the way for personalized orphan drug therapies that are tailored to the individual genetic makeup of patients. This has not only increased the efficacy of treatments but also minimized side effects, making therapies more acceptable for patients suffering from chronic, rare conditions.How Are Changes in Healthcare Dynamics and Patient Needs Influencing Orphan Drugs?
Changing dynamics in healthcare and the evolving needs of patients are influencing the orphan drugs market in multiple ways. There is a rising recognition of the significant unmet medical needs faced by patients suffering from rare diseases, leading to an increasing number of regulatory frameworks aimed at fast-tracking the approval process for these treatments. As a result, regulatory bodies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are increasingly adopting measures to support quicker access to orphan drugs. Moreover, patient advocacy groups have played a key role in driving policy changes and raising awareness, which has, in turn, encouraged more clinical trials and funding in the orphan drug space. This growing awareness is also empowering patients to seek specialized care and treatments, leading to increased diagnosis rates for rare diseases and thus expanding the potential market for orphan drugs.The Growth in the Orphan Drugs Market Is Driven by Several Factors.
The growth in the orphan drugs market is driven by several factors, including government incentives such as extended market exclusivity, grants, and reduced fees that make orphan drug development financially viable for pharmaceutical companies. Advances in biotechnology and genomics have also enabled the development of targeted therapies for rare diseases, increasing the number of potential treatments. The increasing prevalence and diagnosis rates of rare diseases, partly due to improved medical knowledge and awareness, are also contributing to the expansion of the orphan drugs market. Additionally, favorable regulatory frameworks and expedited approval processes from agencies like the FDA and EMA are helping bring orphan drugs to market faster. The role of patient advocacy groups, which actively raise funds, participate in research, and advocate for rare disease patients, is another major growth driver, as they contribute to an environment that supports innovation in orphan drug development.Report Scope
The report analyzes the Orphan Drugs market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Drug Type (Biologics, Non-Biologics); Disease Type (Oncology, Hematology, Neurology, Endocrinology, Cardiovascular, Respiratory, Immunotherapy, Other Disease Types).
- Geographic Regions/Countries: World; USA; Canada; Japan; China; Europe; France; Germany; Italy; UK; Rest of Europe; Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Biologic Drugs segment, which is expected to reach US$308.5 Billion by 2030 with a CAGR of 12.8%. The Non-biologic Drugs segment is also set to grow at 10% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $81 Billion in 2024, and China, forecasted to grow at an impressive 13.1% CAGR to reach $30.2 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Orphan Drugs Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Orphan Drugs Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Orphan Drugs Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AbbVie Inc., AstraZeneca Plc, Biogen., Inc., Bristol-Myers Squibb Company, Daiichi Sankyo Company Limited and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 111 companies featured in this Orphan Drugs market report include:
- AbbVie Inc.
- AstraZeneca Plc
- Biogen., Inc.
- Bristol-Myers Squibb Company
- Daiichi Sankyo Company Limited
- Eisai Co., Ltd.
- F. Hoffmann-La Roche Ltd
- GSK Plc
- Johnson & Johnson
- Novartis AG
- Pfizer Inc.
- Sanofi S.A
- Takeda Pharmaceutical Company Limited
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- AstraZeneca Plc
- Biogen., Inc.
- Bristol-Myers Squibb Company
- Daiichi Sankyo Company Limited
- Eisai Co., Ltd.
- F. Hoffmann-La Roche Ltd
- GSK Plc
- Johnson & Johnson
- Novartis AG
- Pfizer Inc.
- Sanofi S.A
- Takeda Pharmaceutical Company Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 409 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
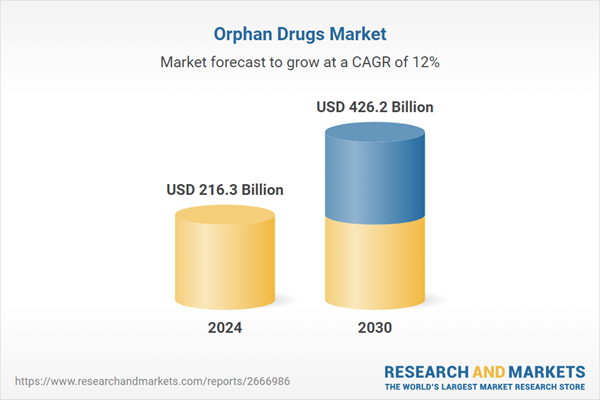
| Estimated Market Value ( USD | $ 216.3 Billion |
| Forecasted Market Value ( USD | $ 426.2 Billion |
| Compound Annual Growth Rate | 12.0% |
| Regions Covered | Global |