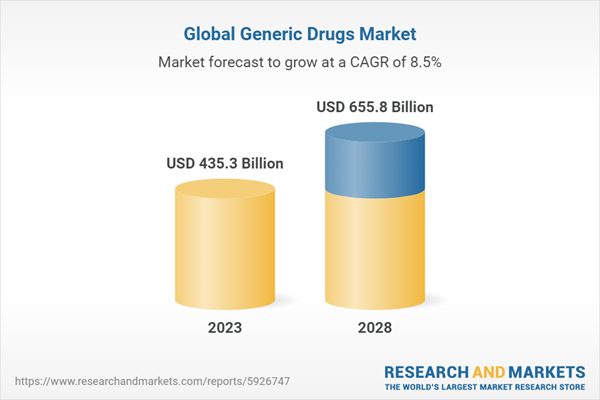
Chapter 2 Summary and Highlights
- Market Outlook
- Market Summary
Chapter 3 Market Overview
- Introduction
- Adequate Market Size
- Patent-Expired Therapies
- Older Products Still Used
- Long-Term Use
- Straightforward Production Technology
- Drugs Used in Primary Care
- Biosimilars
Chapter 4 Market Dynamics
- Market Drivers
- Increasing Patent Cliffs and Loss of Exclusivities
- Favorable Healthcare Policies
- Changing Physician Attitudes
- Market Challenges
- Pricing Pressures and Low Profit Margins
- Patent Extension Strategies of Innovator Companies
Chapter 5 Market Breakdown by Molecule Type
- Global Market for Generic Drugs
- Global Generic Drugs Market, by Molecule Type
- Small Molecule Generics
- Biosimilars
Chapter 6 Market Breakdown by Region
- Introduction
- North America
- U.S.
- Canada
- Europe
- Germany
- France
- Italy
- U.K.
- Spain
- Rest of Europe
- Asia-Pacific
- China
- Japan
- India
- Rest of Asia-Pacific
- Rest of the World
Chapter 7 Sustainability: An ESG Perspective
- Introduction to ESG
- Sustainability in the Generic Drugs Industry: An ESG Perspective
- Key ESG Issues
- Environmental Performance of Key Generic Drug Companies
- Social Performance of Key Generic Drug Companies
- Governance Performance of Key Generic Drug Companies
- Case Study
- Concluding Remarks
Chapter 8 Emerging Trends and Developments
- Key Trends in the Market
- Collaborations for R&D
- Rise of Complex Generics in Immunology and Oncology
- Improving Portfolio Efficiency
- Focus on Emerging Markets
Chapter 9 Regulatory Landscape
- Regulatory Overview
- U.S.
- Evolving Situation in the U.S.
- European Union
- Japan
- Regulation of Biosimilars
- EU Provisions
- Authorized Generic Drugs
Chapter 10 Competitive Landscape
- Competitive Landscape
- M&A Analysis
Chapter 11 Company Profiles
- Aspen Pharmacare
- Aurobindo Pharma
- Cipla Inc.
- Dr. Reddy's Laboratories Ltd.
- Fresenius Kabi
- Lupin Ltd.
- Sandoz
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- Viatris Inc.
List of Tables
Summary Table A: Global Market for Generic Drugs, by Molecule Type, Through 2028
Summary Table B: Global Market for Generic Drugs, by Region, Through 2028
Table 1: List of Block Buster Drugs Undergoing Patent Expiry in Coming Years
Table 2: Global Market for Generic Drugs Compared with the Total Pharmaceutical Market, Through 2028
Table 3: Global Market for Generic Drugs, by Top 10 Countries, Through 2028
Table 4: Global Top Selling Pharmaceuticals, 2022
Table 5: Global Market for Generic Drugs, by Molecule Type, Through 2028
Table 6: Global Market for Small Molecule Generics, by Region, Through 2028
Table 7: Approved Biosimilar Products in the U.S.
Table 8: Global Market for Biosimilars, by Region, Through 2028
Table 9: Global Market for Generic Drugs, by Region, Through 2028
Table 10: North American Market for Generic Drugs, Through 2028
Table 11: U.S. Market for Generic Drugs Compared with the Total Pharmaceutical Market, Through 2028
Table 12: Canadian Key Therapeutic Areas in Retail and Hospital Purchases, 2022
Table 13: Canadian Market for Generic Drugs Compared with the Total Pharmaceutical Market, Through 2028
Table 14: European Market for Generic Drugs, by Country, Through 2028
Table 15: German Market for Generic Drugs Compared with the Total Pharmaceutical Market, Through 2028
Table 16: French Market for Generic Drugs Compared with the Total Pharmaceutical Market, Through 2028
Table 17: Italian Market for Generic Drugs Compared with the Total Pharmaceutical Market, Through 2028
Table 18: U.K. Market for Generic Drugs Compared with the Total Pharmaceutical Market, Through 2028
Table 19: Spanish Market for Generic Drugs Compared with the Total Pharmaceutical Market, Through 2028
Table 20: Penetration of Generic Drugs in the Pharmaceutical Market in Other European Countries, 2022
Table 21: Rest of the European Market for Generic Drugs Compared with the Total Pharmaceutical Market, Through 2028
Table 22: Asia-Pacific Market for Generic Drugs, by Country, Through 2028
Table 23: Chinese Market for Generic Drugs Compared with the Total Pharmaceutical Market, Through 2028
Table 24: Japanese Market for Generic Drugs Compared with the Total Pharmaceutical Market, Through 2028
Table 25: Indian Market for Generic Drugs Compared with the Total Pharmaceutical Market, Through 2028
Table 26: Rest of Asia-Pacific Market for Generic Drugs Compared with the Total Pharmaceutical Market, Through 2028
Table 27: RoW Market for Generic Drugs Compared with the Total Pharmaceutical Market, Through 2028
Table 28: ESG Rankings for Major Generic Drugs Companies, 2023*
Table 29: ESG: Environmental Overview
Table 30: ESG: Social Overview
Table 31: ESG: Governance Overview
Table 32: Teva: ESG Ratings Received, 2022
Table 33: Collaborations for R&D and Licensing Agreements, 2022 and 2023
Table 34: Portfolio Expansion Activities of Key Generic Companies
Table 35: Requirements for NDA and ANDA Applications
Table 36: Leading Generic Drug Companies, by Sales, 2022
Table 37: Comparative Analysis of Top Three Competitors
Table 38: M&A Analysis of Generic Drugs Markets, 2022 and 2023
Table 39: Aspen Pharmacare Holdings Ltd.: Company Snapshot
Table 40: Aspen Pharmacare Holdings Ltd.: Financials, 2021 and 2022
Table 41: Aspen Pharmacare Holdings Ltd: Key Product Portfolio
Table 42: Aspen Pharmacare Holdings Ltd.: Recent Developments, 2023
Table 43: Aurobindo Pharma Ltd.: Company Snapshot
Table 44: Aurobindo Pharma Ltd.: Financials, 2021 and 2022
Table 45: Aurobindo Pharma Ltd.: Recent Developments, 2022
Table 46: Cipla Inc.: Company Snapshot
Table 47: Cipla Inc.: Financials, 2021 and 2022
Table 48: Cipla Inc.: Key Product Launches, by Region, 2022
Table 49: Cipla Inc.: Recent Developments, 2023
Table 50: Dr. Reddy's Laboratories Ltd.: Company Snapshot
Table 51: Dr. Reddy's Laboratories Ltd.: Financials, 2021 and 2022
Table 52: Dr. Reddy's Laboratories Ltd.: Biosimilars Portfolio
Table 53: Dr. Reddy's Laboratories Ltd.: Recent Developments, 2022-2023
Table 54: Fresenius Kabi AG: Company Snapshot
Table 55: Fresenius Kabi AG: Biosimilars Portfolio
Table 56: Fresenius Kabi AG: Biosimilar Products in Pipeline
Table 57: Fresenius Kabi AG: Recent Developments, 2022-2023
Table 58: Lupin Ltd.: Company Snapshot
Table 59: Lupin Ltd.: Financials, 2021 and 2022
Table 60: Lupin Ltd.: Recent Developments, 2023
Table 61: Sandoz Group AG: Company Snapshot
Table 62: Sandoz Group AG: Financials, 2021 and 2022
Table 63: Sandoz Group AG: Product Portfolio
Table 64: Sandoz Group AG: Key Biosimilar Products in Phase III and Registration
Table 65: Sandoz Group AG: Recent Developments, 2023
Table 66: Sun Pharmaceutical Industrial Ltd.: Company Snapshot
Table 67: Sun Pharmaceutical Industrial Ltd.: Financials, 2021 and 2022
Table 68: Teva Pharmaceutical Industrial Ltd.: Company Snapshot
Table 69: Teva Pharmaceutical Industrial Ltd.: Financials, 2021 and 2022
Table 70: Teva Pharmaceutical Industrial Ltd.: Biosimilars Portfolio
Table 71: Teva Pharmaceutical Industrial Ltd.: Recent Developments, 2023
Table 72: Viatris Inc.: Company Snapshot
Table 73: Viatris Inc.: Financials, 2021 and 2022
Table 74: Viatris Inc.: Recent Developments, 2022-2023
List of Figures
Summary Figure A: Global Market for Generic Drugs, by Molecule Type, 2020-2028
Summary Figure B: Global Market for Generic Drugs, by Region, 2020-2028
Figure 1: Generic Drugs Market Dynamics
Figure 2: Generic Drugs Approved in the U.S., 2019-2022
Figure 3: Regulatory Pathways for Small Molecule Generics and Biosimilars
Figure 4: Global Market Shares of Generic Drugs, by Molecule Type, 2022
Figure 5: Global Market for Small Molecule Generics, 2020-2028
Figure 6: Global Market for Biosimilars, 2020-2028
Figure 7: Snapshot of Global Market for Generic Drugs, by Region
Figure 8: Global Market Shares of Generic Drugs, by Region, 2022
Figure 9: Annual Savings from Biosimilars and Generics in the U.S., 2019-2022
Figure 10: European Market Shares of Generic Drugs, by Country, 2022
Figure 11: Asia-Pacific Market Shares of Generic Drugs, by Country, 2022
Figure 12: ESG Pillars
Figure 13: Advantages of ESG for Companies in the Generic Drugs Market
Figure 14: Key Focus Areas in ESG Metrics in the Generics Market
Figure 15: Teva: ESG Indicators, 2022
Figure 16: Emerging Trends in the Generic Drugs Market
Figure 17: Aspen Pharmacare Holdings Ltd.: Financials, 2021 and 2022
Figure 18: Aspen Pharmacare Holdings Ltd.: Market Share, by Business Segment, 2022
Figure 19: Aspen Pharmacare Holdings Ltd.: Revenue Share, by Region, 2022
Figure 20: Aurobindo Pharma Ltd.: Financials, 2021 and 2022
Figure 21: Aurobindo Pharma Ltd.: Revenue Share, by Business Segment, 2022
Figure 22: Aurobindo Pharma Ltd.: ANDA Filings, by Region, FY2022
Figure 23: Cipla Inc.: Financials, 2021 and 2022
Figure 24: Cipla Inc.: Revenue Share, by Business Segment, 2022
Figure 25: Cipla Inc.: ANDAs Pipeline, FY 2022
Figure 26: Dr. Reddy’s Laboratories Ltd.: Financials, 2021 and 2022
Figure 27: Dr. Reddy’s Laboratories Ltd.: Revenue Share, by Business Segment, 2022
Figure 28: Dr. Reddy's Laboratories Ltd.: Global Generics Revenue Share, by Therapeutic Area, 2022
Figure 29: Dr. Reddy's Laboratories Ltd.: Revenue Share, by Country, 2022
Figure 30: Lupin Ltd.: Financials, 2021 and 2022
Figure 31: Lupin Ltd.: Revenue Share, by Business Segment, 2022
Figure 32: Lupin Ltd.: India Revenue Share, by Therapeutic Area, 2022
Figure 33: Timeline of Novartis and Sandoz Separation
Figure 34: Sandoz Group AG: Financials, 2021 and 2022
Figure 35: Sandoz Group AG: Market Share, by Business Segment, 2022
Figure 36: Sandoz Group AG: Revenue Share, by Region, 2022
Figure 37: Sun Pharmaceutical Industrial Ltd.: Financials, 2021 and 2022
Figure 38: Sun Pharmaceutical Industrial Ltd.: Revenue Share, by Business Segment, 2022
Figure 39: Cumulative ANDAs Filed and Approved in the U.S., FY 2019-FY 2023
Figure 40: Teva Pharmaceutical Industrial Ltd.: Financials, 2021 and 2022
Figure 41: Teva Pharmaceutical Industrial Ltd.: Revenue Share, by Region, 2022
Figure 42: Viatris Inc.: Financials, 2021 and 2022
Figure 43: Viatris Inc.: Market Share, by Business Segment, 2022
Figure 44: Viatris Inc.: Revenue Share, by Region, 2022