The clinical trial support services market is expected to register a CAGR of 7.5% during the forecast period.
The COVID-19 pandemic affected almost every sector. It had a substantial effect on the clinical trial support services market worldwide. For instance, a research article published in The Lancet, in February 2021, reported that the COVID-19 pandemic tremendously impacted the market for clinical trials, as there was a rising focus on developing new therapeutics or vaccines to treat the COVID-19 disease. The article also reported that an overall increase in the number of clinical trials was observed both in Europe and the United States in 2020 compared to the previous year. Thus, COVID-19 led to the development of new drugs and vaccines that significantly impacted the growth of the market. However, with the emergence of new infectious and chronic diseases, the demand for clinical trial support services may continue to increase over the forecast period.
The major factors propelling the growth of the market are increasing demand for clinical trials in emerging markets due to the high burden of chronic and infectious diseases, high R&D spending of pharmaceutical companies, an increasing number of contract research organizations, and the prevalence of diseases. The rising prevalence of chronic, infectious, and tropical diseases in emerging countries is creating unmet demand for developing novel drugs for treatment, which requires conducting clinical trials. For instance, an article published by Health World Economic Times, in May 2022, reported that India approved over 100 global clinical trials in 2021, 31.6% higher than the number three years ago. Thus, an increase in clinical trials for new drug molecules is expected to drive the growth of the studied market.
An article published by BMJ Open, in January 2022, reported that the registration of clinical trials on the Pan African Clinical Trials Registry (PACTR) increased Y-o-Y and reached 606 trials in 2020. Hence, with the growing number of clinical trials in the emerging market, the demand for clinical trial support services is expected to increase over the forecast period. The number of individuals suffering from insulin resistance, hyperlipidemia, and obesity is also increasing rapidly in these regions, which may boost clinical trials for effective medications and treatment.
Various organizations are developing clinical data management tools and systems to handle massive volumes of data. For instance, in February 2021, Clinical Solutions launched the Elluminate clinical trial management system that facilitates quicker and more informed decision-making. Thus, such developments to manage the data and services for clinical trials are likely to increase the demand for clinical trial support services, thereby propelling the market's growth.
The R&D spending of pharmaceutical, biopharmaceutical, and medical devices companies has been steadily increasing over the past few years. These companies plan to develop new systems and databases to manage the services and information related to clinical trials, thereby fueling the growth of the clinical trials support services market. For instance, in Pfizer Inc.’s 2021 annual report, it was reported that the company's R&D expenditure has increased over the years, from USD 9,393 million in 2020 to USD 13,829 million in 2021. Therefore, the increasing spending by companies on R&D activities to accelerate the development of drugs through clinical trials is also expected to have a significant impact on the growth of the studied market.
Due to the increasing demand for clinical trials in emerging markets caused by the high burden of chronic and infectious diseases, high R&D spending of pharmaceutical companies, an increasing number of contract research organizations, and the prevalence of diseases, the market is expected to witness significant growth over the forecast period. However, the lack of an adequate regulatory framework for conducting clinical trials in some countries and stringent regulations for patient enrollment may hamper the growth of the studied market.
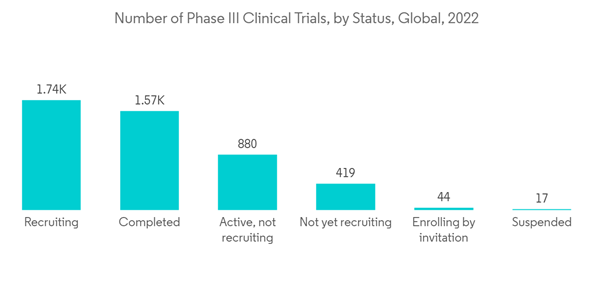
The National Clinical Trial (NCT) Registry data, updated on clinical trial. gov in November 2022, reported that globally, there are around 12,136 clinical trials in the phase III stage for various indications. The high number of ongoing phase III clinical trials is expected to contribute to the segment’s growth during the forecast period.
Strategic partnerships and agreements between key market players are also contributing to the growth of this segment. For instance, in October 2022, Sanofi entered an agreement with the New York-based TrialSpark, which offers a technology-based model for drug development. The partnership will focus on pursuing the acquisition or in-licensing and development of clinical-stage phase II and phase III drug candidates in areas of high and unmet patient needs. Such initiatives of the acquisition of in-licensing activities may lead to the development of more candidates in phase III, driving the segment’s growth.
In June 2022, AION Labs, in partnership with BioMed X, launched the Fourth Global Call for Application: Prediction of Clinical Trial Outcome in Cancer Patient Populations. AION Labs’ fourth start-up will focus on the development of an AI platform that optimizes the patient population for clinical phase III studies by identifying biomarkers within the existing single-arm early phase I/II data. Such platforms are expected to drive the segment’s growth due to the optimization of patients in phase III trials.
Thus, due a high number of clinical trials reported in the phase III segment and the increasing partnerships between key market players, the segment is expected to witness significant growth over the forecast period.
The collaborations, partnerships, and mergers between key market players are driving the growth of the market in the region. For instance, in June 2022, Pfizer, MorphoSys, and Incyte entered a clinical trial collaboration and supply agreement to investigate the immunotherapeutic combination of Pfizer’s TTI-622, a novel SIRPα-Fc fusion protein and Monjuvi (tafasitamab-cxix) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL). Thus, such collaborations are driving the growth of the market in the region.
The prevalence of diseases, such as osteoarthritis, has increased significantly in Canada, which is propelling the growth of the market in the region. For instance, a report commissioned by the Arthritis Community Research and Evaluation Unit (ACREU), Canada, reported that around 15% of Canadians aged 20 years and above had osteoarthritis. The report further stated that more than 4 million Canadians, or about 1 in every 7 adults, have osteoarthritis in Canada. Thus, the high burden of orthopedic disorders is expected to boost the demand for the availability and development of therapeutics or medical devices, ultimately garnering clinical trials for the same, which may fuel the overall market's growth.
The increasing cases of infectious diseases is creating a major healthcare burden in Mexico, which is also consistently driving the growth of the market. For instance, an article published in May 2021 reported that the overall seroprevalence of anti-DENV (dengue virus) IgG at enrolment was 19.4%, with the highest seroprevalence rate observed in the adult group in Mexico. Such instances indicate the high prevalence of dengue in the country, which increases the demand for the development of dengue vaccines following clinical trials.
Thus, the increasing cases of chronic and infectious diseases and increased demand for clinical trials, coupled with partnerships between the key market players in the region, are anticipated to significantly boost the growth of the North American clinical trials support services market during the forecast period.
This product will be delivered within 2 business days.
The COVID-19 pandemic affected almost every sector. It had a substantial effect on the clinical trial support services market worldwide. For instance, a research article published in The Lancet, in February 2021, reported that the COVID-19 pandemic tremendously impacted the market for clinical trials, as there was a rising focus on developing new therapeutics or vaccines to treat the COVID-19 disease. The article also reported that an overall increase in the number of clinical trials was observed both in Europe and the United States in 2020 compared to the previous year. Thus, COVID-19 led to the development of new drugs and vaccines that significantly impacted the growth of the market. However, with the emergence of new infectious and chronic diseases, the demand for clinical trial support services may continue to increase over the forecast period.
The major factors propelling the growth of the market are increasing demand for clinical trials in emerging markets due to the high burden of chronic and infectious diseases, high R&D spending of pharmaceutical companies, an increasing number of contract research organizations, and the prevalence of diseases. The rising prevalence of chronic, infectious, and tropical diseases in emerging countries is creating unmet demand for developing novel drugs for treatment, which requires conducting clinical trials. For instance, an article published by Health World Economic Times, in May 2022, reported that India approved over 100 global clinical trials in 2021, 31.6% higher than the number three years ago. Thus, an increase in clinical trials for new drug molecules is expected to drive the growth of the studied market.
An article published by BMJ Open, in January 2022, reported that the registration of clinical trials on the Pan African Clinical Trials Registry (PACTR) increased Y-o-Y and reached 606 trials in 2020. Hence, with the growing number of clinical trials in the emerging market, the demand for clinical trial support services is expected to increase over the forecast period. The number of individuals suffering from insulin resistance, hyperlipidemia, and obesity is also increasing rapidly in these regions, which may boost clinical trials for effective medications and treatment.
Various organizations are developing clinical data management tools and systems to handle massive volumes of data. For instance, in February 2021, Clinical Solutions launched the Elluminate clinical trial management system that facilitates quicker and more informed decision-making. Thus, such developments to manage the data and services for clinical trials are likely to increase the demand for clinical trial support services, thereby propelling the market's growth.
The R&D spending of pharmaceutical, biopharmaceutical, and medical devices companies has been steadily increasing over the past few years. These companies plan to develop new systems and databases to manage the services and information related to clinical trials, thereby fueling the growth of the clinical trials support services market. For instance, in Pfizer Inc.’s 2021 annual report, it was reported that the company's R&D expenditure has increased over the years, from USD 9,393 million in 2020 to USD 13,829 million in 2021. Therefore, the increasing spending by companies on R&D activities to accelerate the development of drugs through clinical trials is also expected to have a significant impact on the growth of the studied market.
Due to the increasing demand for clinical trials in emerging markets caused by the high burden of chronic and infectious diseases, high R&D spending of pharmaceutical companies, an increasing number of contract research organizations, and the prevalence of diseases, the market is expected to witness significant growth over the forecast period. However, the lack of an adequate regulatory framework for conducting clinical trials in some countries and stringent regulations for patient enrollment may hamper the growth of the studied market.
Key Market Trends
Phase III Segment is Expected to Witness a Significant Growth During the Forecast Period
Clinical trials in phase III test the novel treatment's efficacy and safety to the accepted standard of care. Phase III trials are primarily concerned with proving and confirming the preliminary data from the earlier trials that the drug is a safe, useful, and effective treatment for the specified indication. These phase III clinical trials are done to evaluate the comparative effect of the new medication over the previous medications available or conducted to confirm and expand on safety and effectiveness results from phase I and II trials.The National Clinical Trial (NCT) Registry data, updated on clinical trial. gov in November 2022, reported that globally, there are around 12,136 clinical trials in the phase III stage for various indications. The high number of ongoing phase III clinical trials is expected to contribute to the segment’s growth during the forecast period.
Strategic partnerships and agreements between key market players are also contributing to the growth of this segment. For instance, in October 2022, Sanofi entered an agreement with the New York-based TrialSpark, which offers a technology-based model for drug development. The partnership will focus on pursuing the acquisition or in-licensing and development of clinical-stage phase II and phase III drug candidates in areas of high and unmet patient needs. Such initiatives of the acquisition of in-licensing activities may lead to the development of more candidates in phase III, driving the segment’s growth.
In June 2022, AION Labs, in partnership with BioMed X, launched the Fourth Global Call for Application: Prediction of Clinical Trial Outcome in Cancer Patient Populations. AION Labs’ fourth start-up will focus on the development of an AI platform that optimizes the patient population for clinical phase III studies by identifying biomarkers within the existing single-arm early phase I/II data. Such platforms are expected to drive the segment’s growth due to the optimization of patients in phase III trials.
Thus, due a high number of clinical trials reported in the phase III segment and the increasing partnerships between key market players, the segment is expected to witness significant growth over the forecast period.
North America is Expected to Witness Significant Growth During the Forecast Period
North America is expected to witness significant growth over the forecast period due to the maximum number of pharmaceutical firms based in the United States to conduct most of their business and clinical trials, increasing R&D investments, and growing demand for drug development. The increasing clinical studies in the region due to the presence of key market players are also driving the market's growth. For instance, in June 2022, Walgreens launched a clinical trial service to provide pharmaceutical companies with patient recruitment and enrollment services.The collaborations, partnerships, and mergers between key market players are driving the growth of the market in the region. For instance, in June 2022, Pfizer, MorphoSys, and Incyte entered a clinical trial collaboration and supply agreement to investigate the immunotherapeutic combination of Pfizer’s TTI-622, a novel SIRPα-Fc fusion protein and Monjuvi (tafasitamab-cxix) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL). Thus, such collaborations are driving the growth of the market in the region.
The prevalence of diseases, such as osteoarthritis, has increased significantly in Canada, which is propelling the growth of the market in the region. For instance, a report commissioned by the Arthritis Community Research and Evaluation Unit (ACREU), Canada, reported that around 15% of Canadians aged 20 years and above had osteoarthritis. The report further stated that more than 4 million Canadians, or about 1 in every 7 adults, have osteoarthritis in Canada. Thus, the high burden of orthopedic disorders is expected to boost the demand for the availability and development of therapeutics or medical devices, ultimately garnering clinical trials for the same, which may fuel the overall market's growth.
The increasing cases of infectious diseases is creating a major healthcare burden in Mexico, which is also consistently driving the growth of the market. For instance, an article published in May 2021 reported that the overall seroprevalence of anti-DENV (dengue virus) IgG at enrolment was 19.4%, with the highest seroprevalence rate observed in the adult group in Mexico. Such instances indicate the high prevalence of dengue in the country, which increases the demand for the development of dengue vaccines following clinical trials.
Thus, the increasing cases of chronic and infectious diseases and increased demand for clinical trials, coupled with partnerships between the key market players in the region, are anticipated to significantly boost the growth of the North American clinical trials support services market during the forecast period.
Competitive Landscape
The clinical trials support services market is fragmented and competitive due to the presence of many companies operating globally and regionally. The competitive landscape includes an analysis of a few international and local companies that hold major market shares, including Charles River Laboratories International Inc., Laboratory Corporation of America Holdings, Eli Lilly and Company, Icon PLC, Novo Nordisk AS, Parexel International Corporation, Pfizer Inc., Thermo Fisher Scientific Inc. (PPD Inc.), Iqvia Holdings Inc., F. Hoffmann-La Roche Ltd, Sanofi, and Syneos Health.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
1 INTRODUCTION
4 MARKET DYNAMICS
5 MARKET SEGMENTATION (Market Size by Value - USD million)
6 COMPETITIVE LANDSCAPE
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Charles River Laboratories International Inc.
- Laboratory Corporation of America Holdings
- Eli Lilly and Company
- Icon PLC
- Novo Nordisk AS
- Parexel International Corporation
- Pfizer Inc.
- Thermo Fisher Scientific Inc. (PPD Inc.)
- Iqvia Holdings Inc.
- F. Hoffmann-La Roche Ltd
- Sanofi
- Syneos Health
Methodology

LOADING...