Pharmaceutical Giants Expand Capabilities: WuXi AppTec and Ensoma Lead Acquisitions in Genome Editing Industry
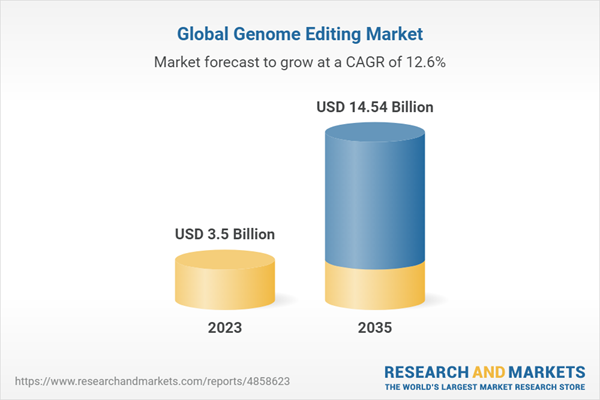
The global genome editing market size for technologies is estimated to be worth USD 3.5 billion in 2023 and is expected to grow at CAGR of 12.6% during the forecast period (2023-2035). Genome editing or gene editing is a genetic engineering technique that enables the modification (insertion, deletion or replacement) of a single gene or a set of genes within the genome of an organism. Over the years, the need for genome editing at the desired site in the genome has resulted in the exploration of various genome editing tools that are being developed by gene editing companies; these include zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs) and CRISPR technology. These gene editing tools have been extensively used as corrective strategies to treat the clinical conditions which develop as a result of genetic abnormalities, such as sickle cell disease, Parkinson’s disease, hearing loss, peripheral artery disease, spinal muscular atrophy, autoimmune diseases, and other genetic disorders.
Amidst the current initiatives to develop targeted gene edited therapies, gene therapy has emerged as a promising option with over 300 candidates in the clinical trials (as of 2023). It is worth highlighting that prophylaxis treatment is another segment of the gene editing applications that has garnered significant attention in the gene editing market. However, the development of gene edited drug products involves a heavy investment for drug discovery, development, and manufacturing in the gene research industry. Further, in order to ensure the efficiency, precision and safe delivery of these drugs, the pharmaceutical companies have been relying novel genome editing technologies, being developed by genome editing companies. This will eventually aid the drug developers to improve the clinical outcome of the therapeutics by achieving the desired genetic manipulation. Given the ongoing pace of innovation in this field, including encouraging results in clinical trials and accelerated drug approvals, the genome editing market is likely to witness significant market growth during the forecast period.
Key Market Insights
The report features an extensive study of the current market landscape, market size and future opportunities associated with the genome editing market (technologies), during the given forecast period. Further, the genome editing market report highlights the efforts of several stakeholders engaged in this rapidly emerging segment of the pharmaceutical industry. Key takeaways of the genome editing market analysis are briefly discussed below.
Genome Editing Market: Current Market Landscape
The genome editing market landscape is concentrated with the presence of over 110 genome editing companies (very large, large, mid-sized and small companies). Of these, 57% of the companies were established post-2016, indicating significant start-up activity in the genome editing industry. Examples of companies (established in 2022 and 2023, in alphabetical order) include Akribion Genomics, CorriXR Therapeutics, Couragene, GENETAGUS, GrittGene Therapeutics, Nvelop Therapeutics, Primera Therapeutics and SE Therapeutics.
Moreover, some of the stakeholders engaged in genome editing market also offer gene editing services in order to optimize the use of their resources and augment their revenue generation opportunities. In Europe, Horizon Discovery (also known as Revvity) emerged as the only genetic engineering company whose business model operates via in-house development of gene edited therapies, licensing of its Pin-point™ base editing platform and through genome editing service capabilities.
Market Segmentation - CRISPR/Cas9 are the Most Sought Genome Editing Tools
Of all the genome editing tools, CRISPR technology, specifically, CRISPR-Cas9 has emerged as the leading genetic engineering technique developed by the stakeholders in the gene editing market. Recently, the domain has witnessed a paradigm shift from conventional CRISPR technology to base editing and prime editing techniques. These novel tools are aimed to improve the long-term safety and efficiency of gene delivery. In this market report, the analyst has captured all the genome editing tools (including CRISPR technology) developed by genome editing companies that are relevant to drug discovery, regenerative medicine and diagnostic applications.
Market Segmentation - North America to Hold the Largest Market Share
Over the past few years, there has been a steady increase in the genome editing companies offering various gene editing technologies. Of these, majority of the technology developers are based in North America. It is worth noting that Intellia Therapeutics, Mammoth Biosciences and Metagenomi (arranged in alphabetical order) are the mid-sized companies based in North America that operate as base editing companies.
Genome Editing Market Trends: Technology Licensing Deals are Driving the Overall Partnership Activity
Several stakeholders have been forging alliances with other industry / non-industry players in the genome editing market for different purposes, including technology licensing, research and development, product licensing and product development. It is worth highlighting that, since 2018, over 250 strategic partnerships have been inked by the technology developers in the genome engineering market. Up lately, the pharmaceutical companies have been engaged in the acquisition of other market players engaged in the genome editing industry in order to expand their capabilities and build a comprehensive pipeline. In March 2023, WuXi AppTec acquired OXGENE and enhanced its capabilities for the development of cell and gene therapies. Earlier, in February 2023, Ensoma acquired Twelve Bio, a gene editing company working in area of the CRISPR medicines. As more drug developers license genome editing technology for evaluation, the market growth is expected to witness favorable market growth during the forecast period.
Top Gene Editing Companies Offering Genome Editing Technologies
Examples of top market players that are engaged in offering genome editing technologies (which have also been profiled in this market report; in alphabetical order) Arcturus Therapeutics, Beam Therapeutics, Caribou Biosciences, Century Therapeutics, CRISPR Therapeutics, Editas Medicine, Graphite Bio, Intellia Therapeutics, Prime Medicine and Vor Biopharma. This market report includes an easily searchable excel database of all the genome editing companies worldwide that offer gene editing tools.
Recent Developments Driving the Market Growth of Genome Editing Market
Driven by numerous developmental breakthroughs and results of studies demonstrating the vast potential of gene therapy and cell therapy in the treatment of various diseases, gene editing tools have garnered considerable attention of various market players engaged in the healthcare industry. Several developments have taken place in the field of genome editing, over the past few years. Some of these recent initiatives have been mentioned below. These developments, even if they took place post the release of the market report, substantiate the overall market trends that the analyst has been outlined in the analyses.
- In October 2023, Intellia Therapeutics announced that the European Medicines Agency (EMA) has granted priority medicine (PRIME) designation to NTLA-2002, an in vivo CRISPR based gene therapy for the treatment of hereditary angioedema.
- In August 2023, Prime Medicine announced a strategic collaboration with Maxcyte for the development of next-generation gene therapies.
- In June 2023, CRISPR Therapeutics and Vertex Pharmaceuticals announced that the United States Food and Drug Administration (USFDA) has accepted the Biologics License Applications (BLA) of exa-cel, an ex vivo CRSIPR based gene edited therapy for the treatment of sickle cell disease and transfusion-dependent β-thalassemia.
- In June 2023, Editas Medicine raised an amount of USD 117.1 million in a secondary offering. It is worth noting that its pipeline candidate EDIT-301 is in early-stage clinical trials for the treatment of sickle cell disease and transfusion-dependent β-thalassemia.
Scope of the Report
The market report presents an in-depth analysis of the various firms / organizations that are engaged in this market, across different segments, as defined below:
- Base Year: 2022
- Forecast Period: 2023-2035
- Market Size 2023: $3.5 billion
- CAGR: 12.6%
- Type of Gene Editing Technique
- CRISPR-Cas System
- TALENs
- Meganucleases
- ZFNs
- Other Techniques
- Type of Therapy
- Cell Therapies
- Gene Therapies
- Other Therapies
- Gene Editing Approach
- Gene Knock-Out Approaches
- Gene Knock- In Approaches
- Gene Delivery Method
- Ex-Vivo Delivery Methods
- In-Vivo Delivery Methods
- Gene Delivery Modality
- Viral Vectors
- Non-Viral Vectors
- Application Area
- Drug Discovery and Development
- Diagnostics
- Type of End User
- Pharmaceutical and Biotechnology Companies
- Academic and Research Institutes
- Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Payment Method Employed
- Upfront Payments
- Milestone Payments
- Key Companies Profiled
- Arcturus Therapeutics
- Avectas
- Beam Therapeutics
- Bio-Sourcing
- Caribou Biosciences
- Century Therapeutics
- CRISPR Therapeutics
- EdiGene
- Editas Medicine
- Flash Therapeutics
- Fortgen
- G+FLAS Life Sciences
- Graphite Bio
- Intellia Therapeutics
- Ntrans Technologies
- OXGENE
- Prime Medicine
- Revvity (formerly known as Horizon Discovery)
- TargetGene Biotechnologies
- Vor Biopharma
- ZeClinics
- Excel Data Packs (Complimentary)
- Technology Landscape Analysis
- Technology Competitiveness Analysis
- Partnerships and Collaborations Analysis
- Patent Analysis
- Funding and Investment Analysis
- Market Forecast and Opportunity Analysis
- Customization Scope: 15% Free Customization Option (equivalent to 5 analysts’ working days)
The genome editing market research report presents an in-depth analysis, highlighting the capabilities of various stakeholders engaged in this market, across different geographies. Amongst other elements, the report includes:
- A preface providing an introduction to the full report, Genome Editing Market: Focus on Technology, 2023-2035.
- An outline of the systematic research methodology adopted to conduct the study on genome editing market (technologies), providing insights on the various assumptions, methodologies, and quality control measures employed to ensure accuracy and reliability of the findings.
- An overview of economic factors that impact the overall genome editing market (technologies), including historical trends, currency fluctuation, foreign exchange impact, recession and inflation measurement.
- An executive summary of the insights captured during the research. It offers a high-level view on the current state of technologies in genome editing industry and its likely evolution in the mid to long term.
- A general overview of genome editing, including a brief discussion on the evolution of this concept over the years. It also includes information on the various genome editing technologies and the application of genome editing in various industries like biotechnology, pharmaceutical and agriculture. Further, it highlights the various emerging genome editing technologies.
- A detailed assessment of the current technology landscape of technologies for genome editing based on several relevant parameters, such as type of gene editing technique (CRISPR-Cas system, meganucleases, TALENs, ZFNs and other techniques), gene editing approach (gene knock-in and gene knock-out), type of gene delivery method (in-vivo and ex-vivo), type of gene delivery modality (cells, viral vectors, non- viral vectors and other modalities), highest phase of drug development supported (discovery, preclinical, clinical and commercial), therapeutic area (oncological disorders, genetic disorders, neurological disorders, hematological disorders, immunological disorders, metabolic disorders, ophthalmological disorders, infectious diseases, cardiovascular disorders, hepatic disorders, muscle-related disorders, autoimmune disorders, pulmonary disorders and other disorders) and application area (drug discovery, regenerative medicine, plant gene editing, and diagnostics). In addition, the chapter features information on various technology developers, along with analysis based on multiple parameters, such as year of establishment, company size, location of headquarters, operational model and most active players (in terms of number of technologies developed).
- An insightful competitiveness analysis of genome editing technologies based on developer strength (in terms of the experience of the developer) and technology strength (in terms of type of gene editing technique, gene editing approach, in-silico (CADD) analysis, type of gene delivery method, type of gene delivery modality, highest phase of development supported and application area).
- Elaborate profiles of the prominent genome editing companies (shortlisted based on a proprietary criterion) developing genome editing technologies. Each profile features a brief overview of the company, details related to its financial information, technology portfolio, recent developments and an informed future outlook. Further, the chapter also includes profiles of other leading players developing genome editing technologies.
- A detailed analysis of the partnerships inked between stakeholders in genome editing market, since 2018, covering technology licensing agreements, research and development agreements, technology integration agreements, mergers and acquisitions, product development agreements and other relevant agreements.
- An in-depth analysis of various patents that have been filed / granted by the technology developers related to genome editing, since 2018, taking into consideration parameters, such as publication year, geographical region, CPC symbols and leading players (in terms of number of patents filled / granted). In addition, the chapter includes a detailed patent benchmarking and an insightful valuation analysis, highlighting the leading patents (in terms of number of citations).
- A detailed analysis of funding and investments raised by genome editing technology developers, based on relevant parameters such as year of funding, type of funding, amount invested, regional distribution, most active players (in terms of number of funding instances and amount raised) and most active investors (in terms of number of funding instances).
- An in-depth analysis of the factors that can impact the growth of genome editing market (technologies). It also features identification and analysis of key drivers, potential restraints, emerging opportunities, and existing challenges.
One of the key objectives of this market report was to estimate the current genome editing market size, opportunity and the future growth potential of the genome editing market (technologies), over the forecast period. the analyst has provided informed estimates on the likely evolution of the market for the forecast period, 2023-2035. the year-wise projections of the current and forecasted opportunity have been further segmented based on relevant parameters, such as type of gene editing technique (CRISPR-Cas System, TALENs, meganucleases, ZFNs and other techniques), type of therapy (cell therapies, gene therapies and other therapies), gene editing approach (gene knock-out approaches and gene knock-in approaches), gene delivery method (ex-vivo and in-vivo), gene delivery modality (viral vectors and non-viral vectors), application area (drug discovery and development, and diagnostics), type of end user (pharmaceutical and biotechnology companies, and academic and research institutes), key geographical regions (North America, Europe and Asia-Pacific) and payment method employed (upfront payments and milestone payments). In order to account for future uncertainties associated with some of the key parameters and to add robustness to the model, the analyst has provided three market forecast scenarios, namely conservative, base and optimistic scenarios, representing different tracks of the market growth.
The opinions and insights presented in the report were influenced by discussions held with stakeholders in this industry. The report features detailed transcripts of interviews held with various industry stakeholders.
All actual figures have been sourced and analyzed from publicly available information forums and primary research discussions. Financial figures mentioned in this report are in USD, unless otherwise specified.
Table of Contents
Companies Mentioned
- 2seventy bio
- 5AM Ventures
- 683 Capital
- AbbVie
- Abcam
- Acrigen Biosciences
- Acuitas Therapeutics
- Affini-T Therapeutics
- Aju IB Investments
- Akribion Genomics
- Aldevron
- Alexion Pharmaceuticals
- Alia Therapeutics
- Allogene Therapeutics
- Ally Bridge Group
- Almirall
- Altitude Life Science Ventures
- Altium Capital
- Amgen Ventures
- Andreessen Horowitz
- AnGes
- Ankarys Therapeutics
- ANLBIO
- APEIRON Biologics
- Apellis Pharmaceuticals
- Applied Biological Materials
- Applied StemCell
- Arbor Biotechnologies
- ARCH Venture Partners
- Arctoris
- Arcturus Therapeutics
- Arix Bioscience
- Armistice Capital
- ArrowMark Partners
- ArsenalBio
- Artisan Bio
- Asahi Kasei Corporate Venture Capital
- Ascenion
- Asklepios BioPharmaceutical
- Astellas Pharma
- AstraZeneca
- ATCC
- ATLATL Innovation Centre
- Avectas
- Avoro Capital Advisors
- Avoro Ventures
- Axcelead
- Axxam
- Bain Capital Life Sciences
- Bayer
- Beam Therapeutics
- Bill & Melinda Gates Foundation
- Bio Palette
- Bio-Sourcing
- Biocytogen
- Biogen
- Bioluminescence Ventures
- Biomedical Advanced Research and Development Authority (BARDA)
- Bioneer
- Blink Therapeutics
- BlueRock Therapeutics
- BOP Capital
- Boston Children's Hospital
- Boxer Capital
- BRAIN Biotech
- BrainXell
- Branca Bunús
- BrightPath Biotherapeutics
- Bristol-Myers Squibb
- Broad Institute of MIT and Harvard
- Brookhaven Bio
- Byers Capital
- C4U
- C4X Discovery
- CaaS Capital
- Cabaletta Bio
- California Institute for Regenerative Medicine (CIRM)
- Canaccord Genuity
- CANbridge Pharmaceuticals
- Capsida Biotherapeutics
- Capstan Therapeutics
- Caribou Biosciences
- Cartherics
- CasCure Therapeutics
- Casdin Capital
- Caszyme
- Catalent
- Catalio Capital Management
- Catamaran Bio
- CCRM
- CD Capital
- Cellecta
- Cellectis
- CellFE
- Celularity
- Center for Breakthrough Medicines
- Central Institute for Experimental Animals (CIEA)
- Century Therapeutics
- CF Foundation
- CHA Biotech
- CHA Research Institute
- Charcot-Marie-Tooth Association
- China Life Healthcare Fund
- ChristianaCare
- Cimeio Therapeutics
- Clairvoyant Ventures
- CMB International Capital Holdings
- Cormorant Asset Management
- CorriXR Therapeutics
- Cosmo Bio
- Cota Capital
- Couragene
- Cowen Healthcare Investments
- CPE
- Creative Biogene
- CRISP-HR Therapeutics
- CRISPR Biotech Engineering
- Crispr Stem & Therapeutics
- CRISPR Therapeutics
- Crown Bioscience
- CSL Seqirus
- Curamys
- Curia
- CyGenica
- Cystic Fibrosis Foundation
- Cytosurge
- Cytovia Therapeutics
- D1 Capital Partners
- Daiichi Sankyo
- Danaher
- DCI Partners
- Decheng Capital
- DefiniGEN
- Delos Capital
- Duke University School of Medicine
- Duke-NUS Medical School
- Dynamk Capital
- EcoR1 Capital
- EdiGene
- Editas Medicine
- eGenesis
- Elaia Partners
- Eledon Pharmaceuticals
- Eli Lilly
- Emendo Biotherapeutics
- Emerson Collective
- Empirica
- Ennovation Ventures
- Ensoma
- EPIQ Capital Group
- ERS Genomics
- ETP Ventures
- Euclidean Capital
- Eurofins
- European Innovation Council (EIC)
- European Investment Bank
- EV Biologics
- Evercrisp Biosciences
- Evotec
- Excision BioTherapeutics
- Exothera
- F-Prime Capital
- Farallon Capital
- FASMAC
- Feldan Therapeutics
- Flash Therapeutics
- Foresite Capital
- Fortgen
- Fresenius Medical Care Ventures (FMCV)
- Friedreich’s Ataxia Research Alliance (FARA)
- FUJIFILM Cellular Dynamics
- Future Fields
- G+FLAS Life Sciences
- GC Cell
- GEcoll Biomedical
- GEMoaB
- GenAhead Bio
- GeneLancet Biosciences
- Genentech
- GENETAGUS
- Genethon
- Genexine
- GenKOre
- GenNBio
- genOway
- GenScript
- German Center for Neurodegenerative Diseases (DZNE)
- Gilead Sciences
- GL Ventures
- GordonMD Global Investments
- Graphite Bio
- GreatPoint Ventures
- Green Cross LabCell
- Green Sands
- Gritstone Oncology
- GrittGene Therapeutics
- GSK Consumer Healthcare
- Guide Therapeutics (a subsidiary of Beam Therapeutics)
- Haihe Laboratory of Cell Ecosystem
- Hamburg Investment and Development Bank
- Hangzhou Huixin Biotechnology
- Hansa Biopharma
- Harvard University
- Hatteras Investment Partners
- HBM Healthcare Investments
- HebeCell
- Heights Capital
- Hera Biolabs
- Hercules Capital
- Hitachi Ventures
- Horizon Technology Finance
- Hunterian Medicine
- IDG Capital
- iECURE
- Illumina
- Immatics
- Immunochina
- In Vivo Biosystems (formerly known as NemaMetrix)
- Inceptor Bio
- Incisive Genetics
- Infinome Biosciences
- Inhibrx
- Innovation Agency
- Innovationsstarter Fonds Hamburg
- Inscripta
- Intellia Therapeutics
- Intima Bioscience
- Invesco Asset Management
- Investissement Québec
- Invus
- Ionis Pharmaceuticals
- Iovance Biotherapeutics
- Irving Investors
- Israeli Ministry of Health
- JAFCO
- Janssen Biotech
- Japan SLC
- Jasper Therapeutics
- Jenthera Therapeutics
- JS Capital Management
- JW Therapeutics
- Karius
- Kite Pharma
- Kite Ventures
- Kiwoom Investment
- Kleiner Perkins
- KRYSP-R
- KSQ Therapeutics
- Kyverna Therapeutics
- Lake Bleu Capital
- Lapam Capital
- Leaps by Bayer
- Lepton Pharmaceuticals
- LG Chem
- Life Edit Therapeutics
- LifeSci Venture Partners
- Lilly Asia Venture
- LogicBio Therapeutics
- Longmen Capital
- Lonza
- Loyal Valley Capital
- Lyell Immunopharma
- LYFE Capital
- MacHall Group
- Macrogen
- Madison Avenue Partners
- Magenta Therapeutics
- Makana Therapeutics
- Mammoth Biosciences
- Matrix Partners China
- Maxcyte
- Mayfield
- Mayo Clinic
- Memorial Sloan Kettering Cancer Center
- Mercia
- Merck
- Metagenomi
- Micro CRISPR
- MidCap Financial
- Mind the Byte
- Mizuho Securities
- Modalis Therapeutics
- Moderna
- Mogrify
- Monashee Investment Management
- Moogene Medi
- Morningside Ventures
- Myeloid Therapeutics
- Myllia Biotechnology (formerly known as Aelian Biotechnology)
- Mérieux Développement
- Nanjing Bioheng Biotech
- National Institutes of Health (NIH)
- National Science Foundation
- Nephrogen
- Neukio Biotherapeutics
- New England Biolabs
- New South Wales Government
- Newpath Partners
- Newtyn Management
- Nippon Gene
- Nkarta
- Noile-Immune Biotech
- Novartis
- Novartis Gene Therapies (formerly known as AveXis)
- Novo Holdings
- Novo Nordisk
- Ntrans Technologies
- NUVISAN ICB
- Nvelop Therapeutics
- Oak HC/FT
- Octagon Capital
- ONK Therapeutics
- Orbital Therapeutics
- Osage University Partners
- Otsuka Pharmaceutical
- Outpace Bio
- Oxford Finance
- OXGENE
- Paladin Capital
- PanCELLa
- Pangen Biotech
- Panmure Gordon
- Pantherna Therapeutics
- Parker Institute for Cancer Immunotherapy (PICI)
- Peking Union Medical College Hospital
- Peking University Cancer Hospital
- Pencil Biosciences
- Perceptive Advisors
- Pfizer
- PFM Health Sciences
- Phen Vista
- Pluristyx
- Polaris Partners
- Polyplus-transfection
- Precision BioSciences
- Prevail Therapeutics
- Prime Medicine
- Primera Therapeutics
- Primordial Genetics
- ProBioGen
- Promega
- Prorenata Biotech
- PROVIREX Genome Editing Therapies
- Qihan Biotech
- QUIDDITAS Therapeutics
- RA Capital Management
- Recipharm
- ReCode Therapeutics
- Recombinetics
- Red Tree Venture Capital
- Redmile Group
- Reforgene Medicine
- Rege Nephro
- Regeneron
- Resilience
- Revvity (formerly known as Horizon Discovery)
- Rewrite Therapeutics
- Ridgeback Capital Investments
- Rock Springs Capital
- RTW Investments
- Rutgers University
- Samsara BioCapital
- Sana Biotechnology
- Sandhill Therapeutics
- Sangamo Therapeutics
- Sanofi
- Santa Cruz Biotechnology
- Sanzheng Health Investment
- Scottish Enterprise
- Scribe Therapeutics
- SDIC Venture Capital
- SE Therapeutics
- Seamus Mulligan
- Seattle Children’s Research Institute
- Sequoia Capital China
- Sestina Bio
- Setsuro Tech
- Shanghai BDgene Technology
- Shoreline Biosciences
- Silicon Valley Bank
- Simcere Pharmaceuticals
- Singapore Economic Development Board (EDB)
- SJ Investment Partners
- SML Genetree
- Sofinnova Partners
- Solana Biosciences
- Songhe Capital
- SOSV
- SparingVision
- Sphere Fluidics
- Spotlight Therapeutics
- SpringWorks Therapeutics
- Starfish Innovations
- Stellate DNA
- STIC Ventures
- Stifel
- Strongest Hearts Foundation
- Sumitomo
- SymBiosis
- Syngulon
- Synthego
- T&R Biofab
- T-MAXIMUM Biotech
- Takara Bio
- Takeda Pharmaceuticals
- TargetGene Biotechnologies
- Taros Chemicals
- TCG Crossover
- TCR² Therapeutics
- TechLife Capital
- Temasek
- Teneobio
- The Wells Investment
- Thermo Fisher Scientific
- Tiziana Life Sciences
- Tofflon
- ToolGen
- TPG Capital
- TransCode Therapeutics
- Transomic Technologies
- TransViragen
- Trentino Invest
- Tsinghua Holdings Capital
- Twelve Bio (a subsidiary of Ensoma)
- Twist Bioscience
- TxCell
- University of Bern
- University of California
- University of California San Francisco
- University of Edinburg
- University of Miami
- University of North Carolina
- University of Pennsylvania
- University of Wisconsin-Madison
- Versant Ventures
- Vertex Pharmaceuticals
- Verve Therapeutics
- Vesigen Therapeutics
- ViaCyte
- Vida Ventures
- Vingroup
- VirEdit Biosciences
- Viromer Transfection
- Virostem Biotechnology
- viTToria Biotherapeutics
- VivaZome Therapeutics
- Vivlion
- Vor Biopharma
- Voyager Therapeutics
- Walking Fish Therapeutics
- Wellington Management
- Western Alliance Bank
- Westlake Village BioPartners
- WuXi AppTec
- Yaotang (Shanghai) Biotechnology
- Yuansheng Venture Capital
- ZeClinics
- Zhejiang University
Methodology

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 270 |
| Published | January 2024 |
| Forecast Period | 2023 - 2035 |
| Estimated Market Value ( USD | $ 3.5 Billion |
| Forecasted Market Value ( USD | $ 14.54 Billion |
| Compound Annual Growth Rate | 12.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 458 |