Free Webex Call
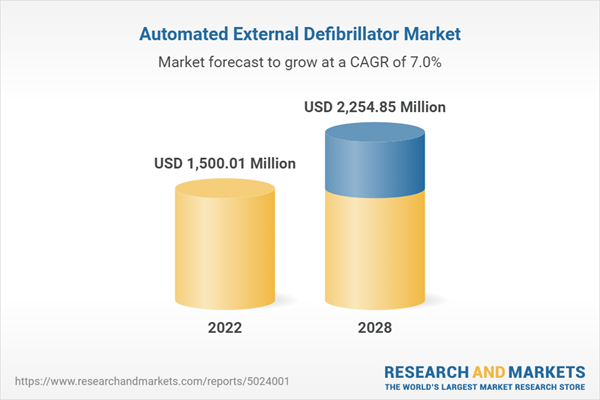
The Global Automated External Defibrillator Market was valued at USD 3,215.2 million in 2022 and is expected to exhibit robust growth in the forecast period, with a CAGR of 7.0% through 2028. Defibrillators, as medical devices, are utilized to administer electric shocks or pulses to the heart in order to restore a regular heartbeat. They are employed to prevent or treat abnormal, slow, or fast heart rhythms known as arrhythmias. Additionally, defibrillators can revive a heart that has suddenly ceased beating. Various designs and operational methods exist for defibrillators. Automated external defibrillators (AEDs) are widely accessible in public places and can be utilized by untrained individuals in emergency situations, potentially saving someone experiencing cardiac arrest. Other types of defibrillators, such as wearable cardioverter defibrillators (WCDs) and implanted cardioverter defibrillators (ICDs), are employed to prevent sudden death in individuals at high risk of life-threatening arrhythmias. Adjusting to having a defibrillator at home may require time and effort, making awareness of potential issues crucial. Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers:
Growing Incidence of Cardiovascular Diseases
The prevalence of cardiovascular diseases remains a significant cause of mortality globally, despite advancements in emergency cardiac care that enhance a patient's chances of survival. Research indicates that approximately 350,000 Americans succumb to heart disease annually, with half of these fatalities occurring unexpectedly outside of medical facilities when the heart ceases to function. Sudden cardiac arrest, characterized by its abrupt onset, accounts for the majority of these untimely deaths. Ventricular fibrillation, an irregular heart rhythm disorder, is typically responsible for triggering sudden cardiac arrest. The automated external defibrillator (AED) is gaining popularity due to its ability to administer electric shocks for treating abnormal heart rhythms. These devices are designed to detect and treat two specific types of cardiac arrhythmias: pulseless ventricular tachycardia and ventricular fibrillation. In the coming years, the global market for AEDs is expected to present revenue-generating opportunities, driven by the increasing use of these devices in treating patients experiencing sudden cardiac arrest. Furthermore, the growing number of individuals dependent on smoking and alcohol consumption could contribute to prosperous market prospects.Increase in Adoption of Advanced Defibrillator Devices
The adoption of advanced defibrillator devices has seen a significant increase. These modern devices are equipped with user-friendly interfaces and automated features, making them accessible to a wider range of healthcare professionals, including non-specialists. This accessibility has been instrumental in emergency situations, where these devices are confidently operated, improving patient care and outcomes. As healthcare spending continues to rise, both in developed and developing countries, there is a growing investment in upgrading medical equipment and technologies. In line with this, hospitals and healthcare facilities are prioritizing the implementation of advanced defibrillators to meet the unique needs of older patients and provide optimal care.The demand for quality medical care has also been on the rise. Timely medical interventions are crucial, especially in critical situations like cardiac arrest. Defibrillators play a vital role in rapid response to life-threatening arrhythmias, significantly improving the chances of successful resuscitation and better patient outcomes. The availability and proper use of defibrillators in healthcare facilities, public spaces, and even homes are prioritized through quality care protocols, leading to higher survival rates. By promptly restoring normal heart rhythms, defibrillators contribute to better patient outcomes and reduced long-term health consequences. This focus on quality care not only enhances patient experience and satisfaction but also drives investments in advanced defibrillator technologies.
Technological advancements in healthcare have paved the way for the development of comprehensive end-to-end solutions in the defibrillator market. Favorable government initiatives, coupled with high healthcare expenditures, have created an environment conducive to innovation. Market participants are actively engaged in integrating innovative hardware and sophisticated software, expanding the scope of pre-hospital care for first responders. Notably, companies like Royal Philips have introduced remote monitoring and defibrillator solutions that enable real-time data transfer, revolutionizing pre-hospital care. Startups also play a significant role in driving market growth through their contributions to the defibrillator industry.
However, the market still faces challenges, including accessibility issues with external defibrillators. Studies have shown that the likelihood of an automated external defibrillator (AED) being nearby during a cardiac arrest is low, and there may be further restrictions on access due to closed buildings. Out-of-hospital cardiac arrest remains a significant cause of fatalities, with low survival rates. The limited use of AEDs in public settings can be attributed to concerns about legal liability, training requirements, limited access, and lack of general awareness. Nonetheless, research has unequivocally demonstrated that immediate access to an AED significantly enhances survival chances.
Strict rules and regulations also pose challenges to the defibrillator market. However, with ongoing advancements and initiatives, the industry continues to progress, aiming to overcome these obstacles and improve healthcare outcomes for patients.
Regulatory authorities established by governments worldwide enforce rigorous regulations and policies to govern the automated external defibrillators market. These measures ensure the accessibility of safe, appropriate, and affordable devices that serve their medical purpose for the public. However, these regulations can present challenges for vendors, resulting in delayed approvals and occasional product recalls that incur significant costs.
The licensing, evaluation, surveillance, and control of medical devices have become increasingly intricate for national regulatory authorities. The growing number of new products, quality concerns, and technical issues arising from rapid scientific advancements pose significant hurdles. Consequently, governments are implementing even more stringent regulations, further impacting market growth.
Key Market Trends
Advancements in Next-Generation Defibrillators
The defibrillator market is projected to experience accelerated growth, surpassing initial expectations, owing to advancements in next-generation defibrillators. Defibrillators play a crucial role in identifying and resolving device-related issues. Sudden Cardiac Arrest (SCA), a leading cause of global mortality, carries potential fatality. However, early intervention and defibrillation can effectively manage this condition. Among external defibrillators, Automated External Defibrillators (AEDs) dominate the market and are expected to witness significant expansion, driven by the increasing number of heart failure patients relying on them. The growing demand aligns with the availability of a wider range of AEDs. Currently, wearables hold a competitive edge over manual external defibrillators. Furthermore, market growth is anticipated to receive a boost from Subcutaneous Implantable Cardioverter-Defibrillators (S-ICDs), MRI-compatible Implantable Cardioverter-Defibrillators (ICDs), and Cardiac Resynchronization Therapy Defibrillators (CRT-Ds) in the upcoming years.Increase in Investments by Market Players
With the advancement of technology and the growing demand for automated external defibrillators, market participants are consistently increasing their investment in the field, propelling the projected growth of the analyzed market in the coming years. MicroPort Cardiac Rhythm Management Limited, a subsidiary of MicroPort Scientific Corporation focused on the development and commercialization of implantable pacemaker and defibrillator equipment, along with associated innovations to manage cardiac rhythm disorders, entered into definitive agreements in July 2021 regarding its USD 150 million Series C financing. Such escalating investment and collaborations by market participants serve as drivers for the expansion of the market.The key players in the automatic external fibrillation market are intensifying their investment in research and development to foster the creation of new products. According to Philips' annual report for 2020, the company incurred research and development expenses amounting to EUR 1,759 million in 2018, EUR 1,884 million in 2019, and EUR 1,915 million in 2020. This increasing investment fuels the expansion of the market.
Segmental Insights
Product Type Insights:In terms of product type, the global defibrillator market is categorized into Manual External Defibrillator, Fully Automated External Defibrillator (AED), Wearable Cardioverter Defibrillator. The AED segment holds a significant market share in 2022. AEDs are portable devices designed to assist individuals in sudden cardiac arrest. An automated external defibrillator is a medical device capable of analyzing the heart's rhythm and delivering an electrical shock or defibrillation to restore an effective rhythm to the heart.
End User Insights
The end user segment is categorized into Hospitals, Pre-Hospitals, Public Access Market, Alternate Care Market, and Home. The Hospitals segment is projected to have a dominant presence in the market due to the higher frequency of cardiac patients receiving care in hospitals and the prevalence of procedures carried out in healthcare facilities. Hospitals employ ICDs and external defibrillators more frequently for the treatment of patients who have experienced sudden cardiac arrest and for other medical purposes. As of January 2021, Boston Scientific reported a worldwide distribution of approximately 659,000 ICDs. This represents a significant increase of 59,000 compared to the 600,000 ICDs distributed by January 2020, indicating a strong demand in the market.Regional Insights:
North America accounted for the highest revenue share in 2022, making it the largest contributor. The significant presence of key players, supportive regulations to enhance the availability of public access AEDs, and the widespread adoption of external defibrillators in healthcare facilities contribute to this dominance. Notably, esteemed organizations like the American Heart Association (AHA) in the U.S. advocate for the implementation of comprehensive Public Access Defibrillation (PAD) programs. These programs emphasize strategic placement of AEDs, regular maintenance and testing, responder training, EMS coordination, and continuous quality improvement.
In contrast, Europe is projected to experience the fastest growth in the external defibrillators market in the coming years. This can be attributed to the well-established healthcare infrastructure, a sizable geriatric population susceptible to cardiovascular events, and the presence of key industry players. A prime example is WEINMANN Emergency Medical Technology GmbH + Co. KG, a German manufacturer renowned for its MEDUCORE Standard series of external defibrillators designed for resuscitation and patient monitoring. These devices are intended for use by emergency medical services personnel, military medical corps, and hospitals.
Key Market Players
- Koninklijke Philips N.V.
- Stryker
- Zoll Medical Corporation
- Nihon Kohden Corporation
- ProgettiSrl
- Schiller AG
- MS Westfalia GmbH
- Bexen Cardio
- Silverline Meditech Pvt. Ltd.
- Mediana Co., Ltd.
Report Scope:
In this report, the Global Automated External Defibrillator Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Automated External Defibrillator Market, By Product Type:
- Manual External Defibrillator
- Fully Automated External Defibrillator
- Wearable Cardioverter Defibrillator
Automated External Defibrillator Market, By End User:
- Hospitals
- Pre-Hospitals
- Public Access Market
- Alternate Care Market
- Home
Automated External Defibrillator Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Automated External Defibrillator Market.Available Customizations:
Global Automated External Defibrillator Market report with the given market data, the publisher offers customizations according to a company's specific needs.This product will be delivered within 1-3 business days.
Table of Contents
1. Product Overview
2. Research Methodology
3. Executive Summary
5. Global Automated External Defibrillator Market Outlook
6. North America Automated External Defibrillator Market Outlook
7. Europe Automated External Defibrillator Market Outlook
8. Asia-Pacific Automated External Defibrillator Market Outlook
9. South America Automated External Defibrillator Market Outlook
10. Middle East and Africa Automated External Defibrillator Market Outlook
11. Market Dynamics
12. Market Trends & Developments
14. Competitive Landscape
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Koninklijke Philips N.V.
- Stryker
- Zoll Medical Corporation
- Nihon Kohden Corporation
- ProgettiSrl
- Schiller AG
- MS Westfalia GmbH
- Bexen Cardio
- Silverline Meditech Pvt. Ltd.
- Mediana Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | September 2023 |
| Forecast Period | 2022 - 2028 |
| Estimated Market Value ( USD | $ 1500.01 Million |
| Forecasted Market Value ( USD | $ 2254.85 Million |
| Compound Annual Growth Rate | 7.0% |
| Regions Covered | Global |