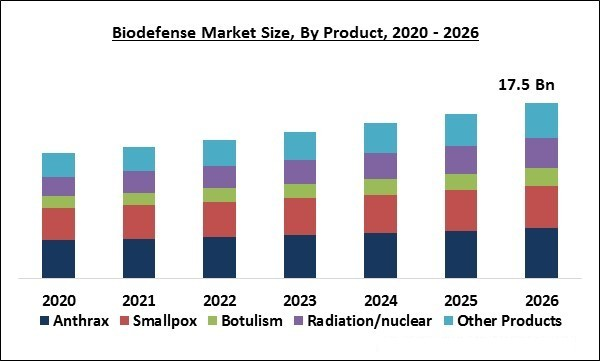
The Global Biodefense Market size is expected to reach $17.5 billion by 2026, rising at a market growth of 5.8% CAGR during the forecast period. Biodefense refers to a group of medical or military steps that are adopted on account of re-establishing the biosecurity of a nation. Threats like biological toxins or infectious agents can be utilized with malicious intentions such as killing or infecting humans, animals, or the environment and to provoke biological warfare. There are various agents that are used for bioterrorism such as living organisms, like bacteria, viruses, fungi, and toxins.
In order to create social and economic turmoil, these agents can be utilized intentionally to infect and kill humans. The biodefense market is fueled by various factors such as beneficial government initiatives, increased government attention and funding towards biodefense strategies. Moreover, rising investment from private companies, and increasing prevalence of different agents like Flu, Ebola Virus, and Zika Virus also widen the scope of biodefense market in the years to come.
Over the last few years, genetic engineering and biotechnology have seen massive technological advancements, which have offered innovative ways to address these deadly and naturally occurring viruses that can be re-designed to create additional harm. Also, there is the easy availability of these organisms, which makes biodefense a significant aspect for countries across the globe. To carry out bioterrorism, various biological agents have been used such as botulism, anthrax, and chemical & nuclear agents, causing severe destruction.
By Product
Based on Product, the market is segmented into Anthrax, Smallpox, Botulism, Radiation/nuclear, and Other Products. The anthrax segment acquired the highest market share in 2019. The segment is anticipated to maintain a considerable CAGR during the forecast years. Anthrax is caused by bacillus anthracis, which is a gram-positive bacterium and it is one of the most preferred weapons against bioterrorism. These spores are largely favored as they can be easily transmitted into the environment by putting them in food, water, powder, and sprays.
By Region
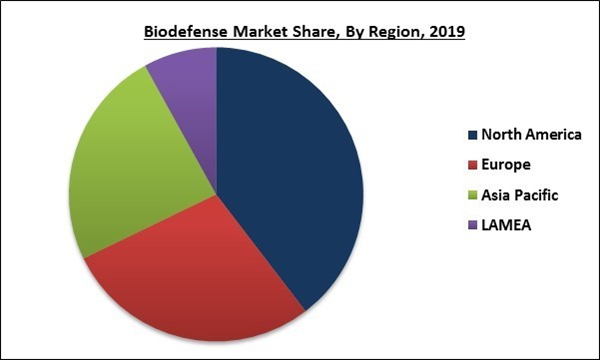
Based on Regions, the market is segmented into North America, Europe, Asia Pacific, and Latin America, Middle East & Africa. North America held the highest revenue share of the biodefense market in 2019. The region is also anticipated to exhibit a significant CAGR during the forecast years owing to the advancements in technology, the presence of massive federal investment, and increasing awareness among the population. Additionally, the presence of the leading players in this region is a positive factor for the market forecast.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Xoma Corporation, Altimmune, Inc., Emergent BioSolutions, Inc., Dynavax Technologies Corporation, SIGA Technologies, Inc., Elusys Therapeutics, Inc., Ichor Holdings, Ltd., Cleveland BioLabs, Inc., Bavarian Nordic A/S and Alnylam Pharmaceuticals, Inc.
Strategies deployed in Bio-defense Market
Jul-2020: Altimmune came into collaboration with DynPort Vaccine Company, a General Dynamics Information Technology company. In this collaboration, they aimed to coordinate with U.S. Government funding efforts and deliver program management, regulatory support for AdCOVID, drug development activity integration, Altimmune’s single-dose intranasal COVID-19 vaccine candidate.
Jun-2020: SIGA Technologies announced the deliveries of oral TPOXX to the U.S. Department of Health and Human Services.
Jun-2020: Ichor Medical Systems signed an agreement with Immunomic Therapeutics, a privately-held clinical-stage biotechnology company. This agreement aimed to support the development of ITI’s investigational plasmid DNA vaccine therapy, ITI-1001.
Apr-2020: Emergent BioSolutions signed a partnership agreement with the U.S. government. The partnership focused on accelerating development of a plasma-derived therapy for patients with COVID-19. Emergent received $14.5 million from the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response (ASPR) at the U.S. Department of Health & Human Services (HHS), to assist its COVID-HIG program, one of two hyperimmune development programs announced by Emergent.
Mar-2020: SIGA Technologies came into collaboration with Turnstone Biologics, a privately-held immuno-oncology company. The collaboration aimed to provide TPOXX in connection with Turnstone’s proprietary SKV vaccinia oncolytic immunotherapy platform. The platform uses vaccinia viruses made for expanded selectivity and safety and high-potency immune stimulation, and offer the delivery of multiple therapeutic agents directly to tumors. The collaboration also offer Turnstone with access to SIGA’s TPOXX oral antiviral capsules for its use in future clinical programs.
Sep-2019: Bavarian Nordic got the approval of JYNNEOS and Monkeypox Vaccine by the U.S. Food and Drug Administration (FDA). JYNNEOS prevents smallpox and monkeypox disease in adults and older, determined to be at high risk for smallpox or monkeypox infection. It is the only approved non-replicating smallpox vaccine in the U.S. and the only approved monkeypox vaccine in the world.
Sep-2019: Emergent BioSolutions received $20 million to develop and manufacture an auto-injector including diazepam to cure nerve agent-induced seizures for U.S. Department of Defense. Emergent’s device aimed for intramuscular buddy-administration to utilize it in military environments and for civilian emergencies.
Jul-2019: Altimmune took over Spitfire Pharma, a California drug developer. Under this acquisition, Spitfire’s flagship product, a treatment for nonalcoholic steatohepatitis, or NASH, a condition marked by fat buildup in the liver integrated with Altimmune’s pipeline of flu and anthrax vaccines.
Jul-2019: SIGA Technologies signed a multi-year contract from the United States Department of Defense (DoD). The contract aimed to assist work necessary to earn a potential label expansion for TPOXX that included Post-Exposure Prophylaxis (PEP) of smallpox.
Mar-2019: Ichor Medical Systems collaborated with AstraZeneca, a British-Swedish multinational pharmaceutical and biopharmaceutical company. The collaboration focused on the development and clinical assessment of plasmid DNA constructs. Moreover Ichor received upfront and annual payments along with development milestones.
Sep-2018: SIGA Technologies entered into a multi-year contract with the Biomedical Advanced Research and Development Authority (BARDA), a part of the U.S. Department of Health and Human Services’ Office of the Assistant Secretary for Preparedness and Response. The contract aimed to deliver oral and intravenous formulations of TPOXX to the Strategic National Stockpile.
Aug-2018: Cleveland BioLabs established a 50-50 joint venture, named Genome Protection, Inc. (GPI). This joint venture is the result of agreement of Cleveland BioLabs and Everon BioSciences, Inc. (Everon). GPI’s aimed to develop and commercialize drugs for anti-aging applications capable of prolonging human health and life-span. Moreover, GPI announced an agreement for Future Equity (SAFE) with venture capital fund Norma Investments Limited (Norma). Under the SAFE, GPI granted Norma the right to purchase shares of GPI’s capital stock in exchange for the payment of up to $30 million, of which $10.5 million was paid shortly after the execution of the SAFE.
Jul-2018: SIGA Technologies announced that its oral TPOXX got the approval from the U.S. Food and Drug Administration (FDA), which treat smallpox to mitigate the impact of a potential outbreak.
Oct-2017: Emergent BioSolutions acquired Sanofi’s ACAM2000 business, which includes ACAM2000, the only smallpox vaccine approved by the U.S. Food and Drug Administration. Under this acquisition, Emergent aimed to assume responsibility for its existing contract with the Centers for Disease Control and Prevention (CDC), offering ACAM2000 to the Strategic National Stockpile (SNS).
Sep-2017: Bavarian Nordic entered into a contract with the Biomedical Advanced Research and Development Authority (BARDA). The contract was focused on providing its freeze-dried Imvamune smallpox vaccine for the U.S. Strategic National Stockpile.
Jul-2017: Emergent BioSolutions signed an agreement with GSK, a leading healthcare company. This agreement aimed to acquire raxibacumab, a fully human monoclonal antibody approved by the U.S. Food and Drug Administration (FDA) for the curing and preventing inhalational anthrax. The acquisition strengthened their leadership position in the market and expanded it franchise of vaccines and therapeutics addressing Category A bioterrorism threats.
Scope of the Study
Market Segmentation:
By Product
By Geography
Companies Profiled
Unique Offerings from the Publisher
In order to create social and economic turmoil, these agents can be utilized intentionally to infect and kill humans. The biodefense market is fueled by various factors such as beneficial government initiatives, increased government attention and funding towards biodefense strategies. Moreover, rising investment from private companies, and increasing prevalence of different agents like Flu, Ebola Virus, and Zika Virus also widen the scope of biodefense market in the years to come.
Over the last few years, genetic engineering and biotechnology have seen massive technological advancements, which have offered innovative ways to address these deadly and naturally occurring viruses that can be re-designed to create additional harm. Also, there is the easy availability of these organisms, which makes biodefense a significant aspect for countries across the globe. To carry out bioterrorism, various biological agents have been used such as botulism, anthrax, and chemical & nuclear agents, causing severe destruction.
By Product
Based on Product, the market is segmented into Anthrax, Smallpox, Botulism, Radiation/nuclear, and Other Products. The anthrax segment acquired the highest market share in 2019. The segment is anticipated to maintain a considerable CAGR during the forecast years. Anthrax is caused by bacillus anthracis, which is a gram-positive bacterium and it is one of the most preferred weapons against bioterrorism. These spores are largely favored as they can be easily transmitted into the environment by putting them in food, water, powder, and sprays.
By Region
Based on Regions, the market is segmented into North America, Europe, Asia Pacific, and Latin America, Middle East & Africa. North America held the highest revenue share of the biodefense market in 2019. The region is also anticipated to exhibit a significant CAGR during the forecast years owing to the advancements in technology, the presence of massive federal investment, and increasing awareness among the population. Additionally, the presence of the leading players in this region is a positive factor for the market forecast.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Xoma Corporation, Altimmune, Inc., Emergent BioSolutions, Inc., Dynavax Technologies Corporation, SIGA Technologies, Inc., Elusys Therapeutics, Inc., Ichor Holdings, Ltd., Cleveland BioLabs, Inc., Bavarian Nordic A/S and Alnylam Pharmaceuticals, Inc.
Strategies deployed in Bio-defense Market
Jul-2020: Altimmune came into collaboration with DynPort Vaccine Company, a General Dynamics Information Technology company. In this collaboration, they aimed to coordinate with U.S. Government funding efforts and deliver program management, regulatory support for AdCOVID, drug development activity integration, Altimmune’s single-dose intranasal COVID-19 vaccine candidate.
Jun-2020: SIGA Technologies announced the deliveries of oral TPOXX to the U.S. Department of Health and Human Services.
Jun-2020: Ichor Medical Systems signed an agreement with Immunomic Therapeutics, a privately-held clinical-stage biotechnology company. This agreement aimed to support the development of ITI’s investigational plasmid DNA vaccine therapy, ITI-1001.
Apr-2020: Emergent BioSolutions signed a partnership agreement with the U.S. government. The partnership focused on accelerating development of a plasma-derived therapy for patients with COVID-19. Emergent received $14.5 million from the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response (ASPR) at the U.S. Department of Health & Human Services (HHS), to assist its COVID-HIG program, one of two hyperimmune development programs announced by Emergent.
Mar-2020: SIGA Technologies came into collaboration with Turnstone Biologics, a privately-held immuno-oncology company. The collaboration aimed to provide TPOXX in connection with Turnstone’s proprietary SKV vaccinia oncolytic immunotherapy platform. The platform uses vaccinia viruses made for expanded selectivity and safety and high-potency immune stimulation, and offer the delivery of multiple therapeutic agents directly to tumors. The collaboration also offer Turnstone with access to SIGA’s TPOXX oral antiviral capsules for its use in future clinical programs.
Sep-2019: Bavarian Nordic got the approval of JYNNEOS and Monkeypox Vaccine by the U.S. Food and Drug Administration (FDA). JYNNEOS prevents smallpox and monkeypox disease in adults and older, determined to be at high risk for smallpox or monkeypox infection. It is the only approved non-replicating smallpox vaccine in the U.S. and the only approved monkeypox vaccine in the world.
Sep-2019: Emergent BioSolutions received $20 million to develop and manufacture an auto-injector including diazepam to cure nerve agent-induced seizures for U.S. Department of Defense. Emergent’s device aimed for intramuscular buddy-administration to utilize it in military environments and for civilian emergencies.
Jul-2019: Altimmune took over Spitfire Pharma, a California drug developer. Under this acquisition, Spitfire’s flagship product, a treatment for nonalcoholic steatohepatitis, or NASH, a condition marked by fat buildup in the liver integrated with Altimmune’s pipeline of flu and anthrax vaccines.
Jul-2019: SIGA Technologies signed a multi-year contract from the United States Department of Defense (DoD). The contract aimed to assist work necessary to earn a potential label expansion for TPOXX that included Post-Exposure Prophylaxis (PEP) of smallpox.
Mar-2019: Ichor Medical Systems collaborated with AstraZeneca, a British-Swedish multinational pharmaceutical and biopharmaceutical company. The collaboration focused on the development and clinical assessment of plasmid DNA constructs. Moreover Ichor received upfront and annual payments along with development milestones.
Sep-2018: SIGA Technologies entered into a multi-year contract with the Biomedical Advanced Research and Development Authority (BARDA), a part of the U.S. Department of Health and Human Services’ Office of the Assistant Secretary for Preparedness and Response. The contract aimed to deliver oral and intravenous formulations of TPOXX to the Strategic National Stockpile.
Aug-2018: Cleveland BioLabs established a 50-50 joint venture, named Genome Protection, Inc. (GPI). This joint venture is the result of agreement of Cleveland BioLabs and Everon BioSciences, Inc. (Everon). GPI’s aimed to develop and commercialize drugs for anti-aging applications capable of prolonging human health and life-span. Moreover, GPI announced an agreement for Future Equity (SAFE) with venture capital fund Norma Investments Limited (Norma). Under the SAFE, GPI granted Norma the right to purchase shares of GPI’s capital stock in exchange for the payment of up to $30 million, of which $10.5 million was paid shortly after the execution of the SAFE.
Jul-2018: SIGA Technologies announced that its oral TPOXX got the approval from the U.S. Food and Drug Administration (FDA), which treat smallpox to mitigate the impact of a potential outbreak.
Oct-2017: Emergent BioSolutions acquired Sanofi’s ACAM2000 business, which includes ACAM2000, the only smallpox vaccine approved by the U.S. Food and Drug Administration. Under this acquisition, Emergent aimed to assume responsibility for its existing contract with the Centers for Disease Control and Prevention (CDC), offering ACAM2000 to the Strategic National Stockpile (SNS).
Sep-2017: Bavarian Nordic entered into a contract with the Biomedical Advanced Research and Development Authority (BARDA). The contract was focused on providing its freeze-dried Imvamune smallpox vaccine for the U.S. Strategic National Stockpile.
Jul-2017: Emergent BioSolutions signed an agreement with GSK, a leading healthcare company. This agreement aimed to acquire raxibacumab, a fully human monoclonal antibody approved by the U.S. Food and Drug Administration (FDA) for the curing and preventing inhalational anthrax. The acquisition strengthened their leadership position in the market and expanded it franchise of vaccines and therapeutics addressing Category A bioterrorism threats.
Scope of the Study
Market Segmentation:
By Product
- Anthrax
- Smallpox
- Botulism
- Radiation/nuclear,
- Other Products
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Companies Profiled
- Xoma Corporation
- Altimmune, Inc.
- Emergent BioSolutions, Inc.
- Dynavax Technologies Corporation
- SIGA Technologies, Inc.
- Elusys Therapeutics, Inc.
- Ichor Holdings, Ltd.
- Cleveland BioLabs, Inc.
- Bavarian Nordic A/S
- Alnylam Pharmaceuticals, Inc.
Unique Offerings from the Publisher
- Exhaustive coverage
- Highest number of market tables and figures
- Subscription based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Chapter 1. Market Scope & Methodology
Chapter 2. Market Overview
Chapter 3. Competition Analysis - Global
Chapter 4. Global Biodefense Market by Product
Chapter 5. Global Biodefense Market by Region
Chapter 6. Company Profiles
Companies Mentioned
- Xoma Corporation
- Altimmune, Inc.
- Emergent BioSolutions, Inc.
- Dynavax Technologies Corporation
- SIGA Technologies, Inc.
- Elusys Therapeutics, Inc.
- Ichor Holdings, Ltd.
- Cleveland BioLabs, Inc.
- Bavarian Nordic A/S
- Alnylam Pharmaceuticals, Inc.
Methodology

LOADING...