On the other hand, after the declining sales from 2016 to 2019, the sales of Adalimumab in the Chinese market have increased by about 7 times in 2020 compared to the previous year. The substantial increase is mainly due to the improvement of medical insurance policies and the rectification of the abuse of adjuvant drugs. Besides, more generic drugs appear in the market since the patent of HUMIRA is about to expire. Although Abbvie Inc. successfully postponed the threat of US biosimilars to 2023, it lost its patent protection in Europe on October 16, 2018. In China, 28 companies have filed generic drug applications for Adalimumab biosimilars in 2019. In 2020, three companies have launched generic drugs, namely Zhejiang Hisun Pharmaceutical Co., Ltd., Bio- Thera Solutions, Ltd., and Innovent Biologics (Suzhou) Co., Ltd.
According to the analyst’s market research, in China, the sales of Adalimumab increased significantly in 2020, reaching approximately CNY 101.11 million (2019 sales revenue is CNY 14.15 million), and the CAGR is around 38% from 2016 to 2020. Therefore, the analyst analyzes that with the emergence of generic drugs, the choice of drugs increases. Therefore, the price of drugs will decrease. Coupled with the strict control of adjuvant drugs and the improvement of medical insurance policies, Adalimumab is expected to be more popular in China in 2021-2025, and sales will also increase accordingly.
Topics Covered:
- The impact of COVID-19 on China's adalimumab market
- Sales value and volume of China's adalimumab 2016-2020
- Competitive landscape of China's adalimumab market
- Prices of adalimumab in China
- Prices of adalimumab in China by regions and manufacturers
- Analysis of factors affecting the development of China's adalimumab market
- Prospect of China's adalimumab market from 2021 to 2025
Table of Contents
Companies Mentioned
- AbbVie Ltd.
- Zhejiang Hisun Pharmaceutical Co., Ltd.
- Bio-Thera Solutions, Ltd.
- Innovent Biologics (Suzhou) Co., Ltd.
Methodology
Background research defines the range of products and industries, which proposes the key points of the research. Proper classification will help clients understand the industry and products in the report.
Secondhand material research is a necessary way to push the project into fast progress. The analyst always chooses the data source carefully. Most secondhand data they quote is sourced from an authority in a specific industry or public data source from governments, industrial associations, etc. For some new or niche fields, they also "double-check" data sources and logics before they show them to clients.
Primary research is the key to solve questions, which largely influence the research outputs. The analyst may use methods like mathematics, logical reasoning, scenario thinking, to confirm key data and make the data credible.
The data model is an important analysis method. Calculating through data models with different factors weights can guarantee the outputs objective.
The analyst optimizes the following methods and steps in executing research projects and also forms many special information gathering and processing methods.
1. Analyze the life cycle of the industry to understand the development phase and space.
2. Grasp the key indexes evaluating the market to position clients in the market and formulate development plans
3. Economic, political, social and cultural factors
4. Competitors like a mirror that reflects the overall market and also market differences.
5. Inside and outside the industry, upstream and downstream of the industry chain, show inner competitions
6. Proper estimation of the future is good guidance for strategic planning.

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 40 |
| Published | April 2021 |
| Forecast Period | 2016 - 2025 |
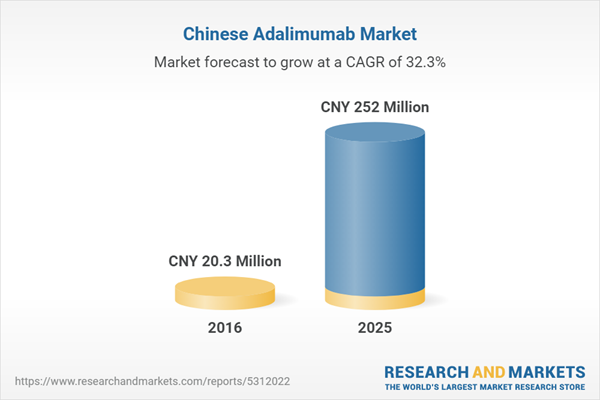
| Estimated Market Value ( CNY | CNY 20.3 Million |
| Forecasted Market Value ( CNY | CNY 252 Million |
| Compound Annual Growth Rate | 32.3% |
| Regions Covered | China |
| No. of Companies Mentioned | 4 |