Report’s Key Features
- PDF with > 130 slides
- Excel file > 2440 patent families + hyperlink to updated online database (legal status, documents etc.)
- IP trends, including time-evolution of published patents, and countries of patent filings
- Patents’ legal status
- Ranking of main patent assignees
- Key players’ IP position and relative strength of their patent portfolios
- Summary of the IP related to the applications: infectious diseases, cancer, cardiovascular, nervous system or prenatal.
- Summary of the IP related to the technologies: extraction or detection and isolation.
- Analysis of patent litigations and review of key patents
Circulating nucleic acids tests, an essential step in prognosis and personalized treatment
In clinical practice today, one of the most eagerly awaited technologies is the liquid biopsy, and, more specifically, the analysis of circulating DNA, which is a safer alternative to solid biopsy. It is a useful and essential element for achieving personalized medicine. It is also a minimally invasive approach for diagnosing or prognosing a disease through markers (e.g. mutations) in body fluids such as blood, urine, saliva, cerebrospinal fluid or pleural fluid. Circulating DNA/RNA analysis is not restricted to oncology, though the research and needs in this area are immense and eagerly awaited. This technology is also applicable to prenatal diagnostics, cardiovascular and nervous system diseases or transplantation. In addition, the development of technologies to intercept and analyze these circulating nucleic acids steeply increased in the last 10 years, as did the number of companies supplying them (e.g. Grail, Guardant Health, Roche, Illumina). In the cancer area, great progress has been made in the last 5 years, since 3 medical devices have arrived on the market and have been FDA approved: the cobas® EGFR Mutation Test and FoundationOne® Liquid CDx by Roche; and the Guardant360® CDx by Guardant Health. The FDA has also granted three Breakthrough Device Designations for a Natera test. At the same time, the number of clinical studies observing circulating nucleic acids continues to increase, reflecting the growing interest in this topic as well as the economic stakes for companies. Despite this, multiple issues are delaying the widespread incorporation of ctDNA into routine patient care. Future focus should be on establishing optimal techniques for sample collection, cfDNA isolation and analysis. In addition, further studies are required to better understand the biological characteristics of cfDNA.
In terms of intellectual property (IP), circulating DNA/RNA is of interest to big companies and start-ups alike, and it is crucial to understand the IP position and strategy of these different players. Indeed, significant numbers of litigations and oppositions have been filed recently, showing the strategic role of IP in this area. Such knowledge can help detect business risks and opportunities, anticipate emerging technologies, and enable strategic decisions to strengthen one’s market position.
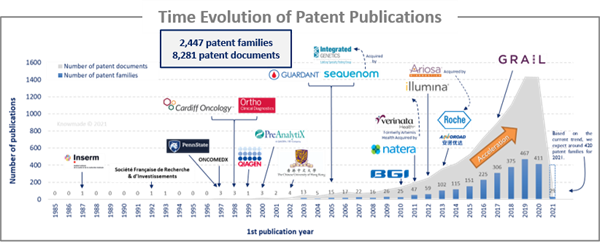
The analysis of the time evolution of patent publications shows that Circulating DNA/RNA started to be investigated in the late 1980s with a first patent published in 1987 by a French Academic player, Inserm. Until 2009, there was a latent period, although highly specialized companies in the field started to emerge, such as CLS Therapeutics, Sequenom (acquired by Integrated Genetics) and OncoMEDx. Some academics also started to take an interest in this field of research, such as American universities (Pennsylvania and John Hopkins) and Chinese universities. Since 2010, an acceleration in patenting activity has been observed, with the arrival of big newcomers such as Roche, Natera, Illumina and BGI.
Analysis by segment
Circulating DNA/RNA has been investigated and the selected patent families labeled according to applications or technologies to which they relate.
This IP landscape features the following 2 types of segmentation:
- Applications: This segmentation includes oncology, prenatal diagnostics, infectious diseases, transplantation, nervous system and cardiovascular system. cfDNA was mainly explored as a diagnostic or prognostic marker and for monitoring treatment efficacy or residual disease.
- Technologies: This segmentation includes Extraction & Preparation of circulating DNA/RNA and the Detection & Analysis of these cfDNA/RNA.
Oppositions and litigations
There is a significant number of EP oppositions (>30) and US litigations for patent infringement (>20), which reflects the strategic issues of circulating DNA/RNA for companies. Most of the proceedings are recent, filed in the past 3 years, and are still pending.
Identifying the companies that have recently emerged in the IP landscape
Among the players owning patent families related to circulating DNA/RNA, over 100 newcomers were identified. These companies are either established companies or start-up firms developing their first methods and/or products in the cell-free DNA/RNA field. These technologies are mainly related to cancer or prenatal diagnostics. Numerous IP newcomers are based in the US and in Asia while some are based in Europe. It is possible that one of these innovative companies could become one of the next healthcare unicorns that the big corporations will be tempted to acquire.
Key patent analysis
This IP study includes a selection and description of key patents. The key patent analysis includes the legal status of the family for each of the main territories, the number of received citations, the review of the main claim(s), the description of interesting features about the innovation disclosures and relevant figures illustrating how the innovation works. The description also contains information about litigation over the patent family in the USA.
Useful Excel patent database
Moreover, the report also includes an Excel database with the >2,240 patents analyzed in this study. This useful patent database allows for multi-criteria searches and includes patent publication numbers, hyperlinks to the original documents, priority dates, titles, abstracts, patent assignees, each patent’s current legal status and segmentation.
Table of Contents
Samples

LOADING...
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Annoroad
- Aviir
- BGI
- Bluestar Genomics
- Cardiff Oncology
- Caredx
- Caris Life Sciences
- Cleveland Heartlab
- Eisai
- Grail
- Guardant Health
- Illumina
- Integrated Genetics (Sequenom)
- Natera
- Oncomedx
- Personal Genome Diagnostics
- Preanalytix
- Qiagen
- Ravgen
- Roche
- Streck
Methodology
The search strategy combines keyword-based searches with expert review and manual screening of the patents. Keywords are searched in the title, abstract, claims and description. Relevant IPC (International Patent Classification) and CPC (Cooperative Patent Classification) are also used for the patent search. The dataset of identified patents is manually cleaned of non-relevant patents. Citing and cited patents of selected relevant patents are also included in the search strategy. The search strategy (keywords and search equations) and the dataset of selected patents is provided in our reports.
The publisher's analysts (PhD team) combine their technical and patent expertise with powerful analytics tools and proprietary methodologies to deliver the most relevant patent analyses.

LOADING...