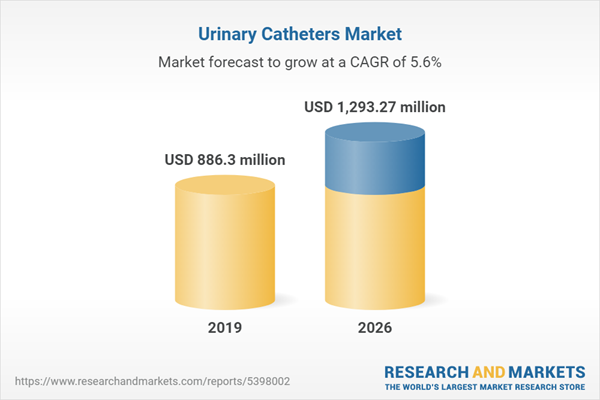
The global urinary catheters market is expected to grow at a compound annual growth rate of 5.55% over the forecast period to reach a market size of US$1,293.272 million in 2026 from US$886.302 million in 2019.
Urinary catheters are used for patients encountering urinary incontinence, urinary maintenance, and other urological messes. A urinary catheter is an adaptable cylinder used to deplete the bladder and amass urine in a seepage pack, which is regularly embedded into the patient's bladder by a clinical expert. A urinary catheter can either be implanted through a urethral catheter or a little opening made lower mid-district (suprapubic catheter). Urinary catheters incorporate inhabiting catheters, discontinuous catheters, and outer catheters. An inhabiting catheter is a versatile cylinder with lumens at two finishes. This kind of urinary catheter is inserted into the bladder through the urethra. A discontinuous catheter is a versatile catheter used to release the bladder at standard stretches. Outer or condom urinary catheter is ordinarily used for more seasoned male patients encountering dementia. The outer urinary catheter is shaped like a condom and is set over the male genital, with a cylinder prompting a seepage sack, which gathers urine.
Presently, considering the utilization cases for urinal catheters, organizations across the globe are putting resources into the innovative work exercises to foster urinary catheters utilizing biocompatible material. The utilization of silicone to foster urinary catheters is additionally expanding as it is heartless toward changes in temperature and impervious to synthetic compounds. Urinary catheters made utilizing silicone additionally decrease the odds of mastitis, urethritis, and trigonites. The most generally utilized materials are latex and silicone. In any case, latex has been found to cause contaminations and responses, thus, its utilization in creating urinary catheters have been decreased. In addition, catheters caused by utilizing silicone don't need to be changed as often as possible when contrasted with the latex ones. Significant associations are acquainting new things withhold their places in the overall market for urinary catheters. These new urinary catheter item dispatches incorporate the usage of better materials that have biocompatibility. Silicone foley urinary catheter is supported as it has a longer life and limits the odds of irritation or pollution.
Additionally, the urinal catheter market is estimated to experience significant growth in the upcoming years owing to the rising geriatric population as well as the rising prevalence of urinary incontinence, which can be defined as the unintentional passing of urine. The per capita healthcare expenditure is currently following an uptrend across developed and developing economies, which has made it possible to implement quality patient care treatment options. Government and regulatory bodies, and more specifically in developing countries are adopting several cost-containment measures to reduce the healthcare burden. The value-based healthcare system helps companies to achieve maximum value for money invested in healthcare, and thus improve outcomes through integrated care pathways, but at the same time is imposing pricing pressure on the manufacturing companies and affecting investments in R&D of innovative medical treatment technologies. Lastly, taking into account the estimated increase in demand for urinal catheters in the upcoming years, market players all around the globe are undertaking various strategic actions to develop new products and subsequently, increase their market share too. For instance, in December 2020, Ingenion Medical Ltd. received about GBP 100,000 funding from the Innovative UK for the development of CymActive urinary catheter, a novel medical device that is designed for treating male patients suffering from urinary retention.
The high prevalence of urinary incontinence is a major factor propelling the demand for urinary catheters, globally. Prevalence rates are higher in the elderly population and among nursing home patients. The prevalence of urinary incontinence is expected to grow due to the growing aging population and the increasing incidence of obesity, as these are the two major risk factors for urinary incontinence further. According to National Kidney Foundation, US, UTI is responsible for nearly 10 million doctor visits each year. Also, according to a report published by the NCBI in December 2019, UTIs are the most common outpatient infections in the United States. With the exception of a spike in young women aged 14−24 years old, the prevalence of UTIs increases with age. The prevalence in women over 65 years of age is approximately 20%, compared with approximately 11% in the overall population. Between 50% and 60% of adult women will have at least one UTI in their life, and close to 10% of postmenopausal women indicate that they had a UTI in the previous year. Thus, with an increase in the number of UTIs occurring every year, the demand for urinal catheters is also expected to increase laterally within the forecast period.
There is an increased risk of securing catheter-related urinary parcel diseases, which ruins the development of this market. As per the Centre for Disease Control and Prevention, roughly 12-16% of emergency clinic patients have an inhibiting urinary catheter during their hospitalization, during which period, a patient has almost 3-7% expanded danger of gaining CAUTI. This may prompt different difficulties like prostatitis, epididymitis, and orchitis, trailed by longer clinic stay and expanded expense and mortality, hence expected to fundamentally block the urinal catheters market development.
Impact of COVID – 19.
Urinary catheters utilized for careful applications are required to be affected somewhat because of the transitory delay of different elective medical procedures, as governments and specialists try to guarantee the accessibility of assets for COVID-19 patients. With an end goal to lessen the strain on the medical care framework, decline infection transmission, and ration individual defensive hardware (PPE), different governments have given rules on elective medical procedures. For example, in March 2020, the US CMS declared that every single elective medical procedure, just as insignificant clinical, careful, and dental techniques, would be postponed, which is relied upon to adversely affect the urinal catheters market development, However, organizations are probably going to restore and continue regularity once limitations on elective medical procedures and development are lifted.
The increasing demand for urinal catheters has led to the entry of several new market players like Bactiguard Holding in the urinal catheters market. The entry of these new players in a market where traditional pharma behemoths like B. Braun Melsungen AG and Boston Scientific Corporation already exist is expected to lead to further innovation in the urinal catheters market. Moreover, in order to further increase their clientele as well as increase their market share in the upcoming years, many of these market players have taken various strategic actions like partnerships and development of novel solutions which primarily focus on providing comfort to the patient, which is expected to keep the market competitive and constantly evolving.
Urinary catheters are used for patients encountering urinary incontinence, urinary maintenance, and other urological messes. A urinary catheter is an adaptable cylinder used to deplete the bladder and amass urine in a seepage pack, which is regularly embedded into the patient's bladder by a clinical expert. A urinary catheter can either be implanted through a urethral catheter or a little opening made lower mid-district (suprapubic catheter). Urinary catheters incorporate inhabiting catheters, discontinuous catheters, and outer catheters. An inhabiting catheter is a versatile cylinder with lumens at two finishes. This kind of urinary catheter is inserted into the bladder through the urethra. A discontinuous catheter is a versatile catheter used to release the bladder at standard stretches. Outer or condom urinary catheter is ordinarily used for more seasoned male patients encountering dementia. The outer urinary catheter is shaped like a condom and is set over the male genital, with a cylinder prompting a seepage sack, which gathers urine.
Presently, considering the utilization cases for urinal catheters, organizations across the globe are putting resources into the innovative work exercises to foster urinary catheters utilizing biocompatible material. The utilization of silicone to foster urinary catheters is additionally expanding as it is heartless toward changes in temperature and impervious to synthetic compounds. Urinary catheters made utilizing silicone additionally decrease the odds of mastitis, urethritis, and trigonites. The most generally utilized materials are latex and silicone. In any case, latex has been found to cause contaminations and responses, thus, its utilization in creating urinary catheters have been decreased. In addition, catheters caused by utilizing silicone don't need to be changed as often as possible when contrasted with the latex ones. Significant associations are acquainting new things withhold their places in the overall market for urinary catheters. These new urinary catheter item dispatches incorporate the usage of better materials that have biocompatibility. Silicone foley urinary catheter is supported as it has a longer life and limits the odds of irritation or pollution.
Additionally, the urinal catheter market is estimated to experience significant growth in the upcoming years owing to the rising geriatric population as well as the rising prevalence of urinary incontinence, which can be defined as the unintentional passing of urine. The per capita healthcare expenditure is currently following an uptrend across developed and developing economies, which has made it possible to implement quality patient care treatment options. Government and regulatory bodies, and more specifically in developing countries are adopting several cost-containment measures to reduce the healthcare burden. The value-based healthcare system helps companies to achieve maximum value for money invested in healthcare, and thus improve outcomes through integrated care pathways, but at the same time is imposing pricing pressure on the manufacturing companies and affecting investments in R&D of innovative medical treatment technologies. Lastly, taking into account the estimated increase in demand for urinal catheters in the upcoming years, market players all around the globe are undertaking various strategic actions to develop new products and subsequently, increase their market share too. For instance, in December 2020, Ingenion Medical Ltd. received about GBP 100,000 funding from the Innovative UK for the development of CymActive urinary catheter, a novel medical device that is designed for treating male patients suffering from urinary retention.
Growth Factors.
- Rising prevalence of urinary tract infections (UTI).
The high prevalence of urinary incontinence is a major factor propelling the demand for urinary catheters, globally. Prevalence rates are higher in the elderly population and among nursing home patients. The prevalence of urinary incontinence is expected to grow due to the growing aging population and the increasing incidence of obesity, as these are the two major risk factors for urinary incontinence further. According to National Kidney Foundation, US, UTI is responsible for nearly 10 million doctor visits each year. Also, according to a report published by the NCBI in December 2019, UTIs are the most common outpatient infections in the United States. With the exception of a spike in young women aged 14−24 years old, the prevalence of UTIs increases with age. The prevalence in women over 65 years of age is approximately 20%, compared with approximately 11% in the overall population. Between 50% and 60% of adult women will have at least one UTI in their life, and close to 10% of postmenopausal women indicate that they had a UTI in the previous year. Thus, with an increase in the number of UTIs occurring every year, the demand for urinal catheters is also expected to increase laterally within the forecast period.
Restraints.
- Complications associated with catheterization.
There is an increased risk of securing catheter-related urinary parcel diseases, which ruins the development of this market. As per the Centre for Disease Control and Prevention, roughly 12-16% of emergency clinic patients have an inhibiting urinary catheter during their hospitalization, during which period, a patient has almost 3-7% expanded danger of gaining CAUTI. This may prompt different difficulties like prostatitis, epididymitis, and orchitis, trailed by longer clinic stay and expanded expense and mortality, hence expected to fundamentally block the urinal catheters market development.
Impact of COVID – 19.
Urinary catheters utilized for careful applications are required to be affected somewhat because of the transitory delay of different elective medical procedures, as governments and specialists try to guarantee the accessibility of assets for COVID-19 patients. With an end goal to lessen the strain on the medical care framework, decline infection transmission, and ration individual defensive hardware (PPE), different governments have given rules on elective medical procedures. For example, in March 2020, the US CMS declared that every single elective medical procedure, just as insignificant clinical, careful, and dental techniques, would be postponed, which is relied upon to adversely affect the urinal catheters market development, However, organizations are probably going to restore and continue regularity once limitations on elective medical procedures and development are lifted.
Competitive Insights.
The increasing demand for urinal catheters has led to the entry of several new market players like Bactiguard Holding in the urinal catheters market. The entry of these new players in a market where traditional pharma behemoths like B. Braun Melsungen AG and Boston Scientific Corporation already exist is expected to lead to further innovation in the urinal catheters market. Moreover, in order to further increase their clientele as well as increase their market share in the upcoming years, many of these market players have taken various strategic actions like partnerships and development of novel solutions which primarily focus on providing comfort to the patient, which is expected to keep the market competitive and constantly evolving.
Market Segmentation:
By Catheter Type
- Indwelling Catheters
- External Catheters
- Intermittent Catheters
By Application
- Urinary Incontinence
- Benign Prostatic Hyperplasia
- Spinal Cord Injuries
- General Surgery
By Geography
- North America
- USA
- Canada
- Mexico
- South America
- Brazil
- Argentina
- Others
- Europe
- UK
- France
- Germany
- Italy
- Others
- Middle East and Africa
- Israel
- Saudi Arabia
- UAE
- Others
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Taiwan
- Thailand
- Indonesia
- Others
Table of Contents
1. Introduction
2. Research Methodology
3. Executive Summary
4. Market Dynamics
5. Urinary Catheters Market Analysis, By Catheter Type
6. Urinary Catheters Market Analysis, By Application
7. Urinary Catheters Market Analysis, By Geography
8. Competitive Environment and Analysis
9. Company Profiles.
Companies Mentioned
- B. Braun Melsungen AG
- Boston Scientific Corporation
- Medtronic
- Coloplast Corp
- ConvaTec Inc.
- Cook Medical
- C. R. Bard, Inc.
- Teleflex Incorporated
- Bactiguard Holding
- Hollister Incorporated
Methodology

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 114 |
| Published | July 2021 |
| Forecast Period | 2019 - 2026 |
| Estimated Market Value ( USD | $ 886.3 million |
| Forecasted Market Value ( USD | $ 1293.27 million |
| Compound Annual Growth Rate | 5.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |