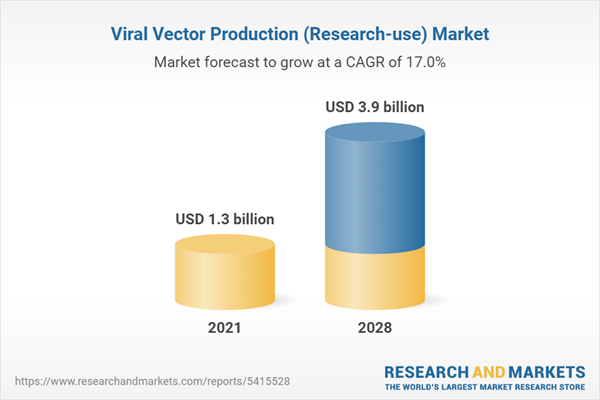
The global viral vector production (research-use) market size is expected to reach USD 3.9 billion by 2028. The market is expected to expand at a CAGR of 18.8% from 2021 to 2028. The expanding research base for advanced therapies has primarily driven the market for research applications including preclinical, clinical, and investigational studies.
The outbreak of the COVID-19 pandemic has created lucrative opportunities for the market players, particularly in the vaccine manufacturing sector. The application of viral vectors in vaccine development against SARS-CoV-2 has witnessed remarkable growth in the fiscal year 2020. By far, the manufacturing of viral vectors for research use is impeded by a lack of production capacity to fulfill the growing market needs.
Thus, operating key stakeholders are engaged in implementing new approaches to overcome these challenges and expand production capacities. The improving ratio of clinical success to the number of clinical trials of gene and cell therapy products is a testament to the enhancing manufacturing capabilities.
Currently, several new gene therapy products are in the late stages of development and the pipeline continues to expand across the globe. The forward momentum for the advanced therapy arena is anticipated to drive investment to conduct research for the development of safe viral vectors and therapies.
This product will be delivered within 1-3 business days.
The outbreak of the COVID-19 pandemic has created lucrative opportunities for the market players, particularly in the vaccine manufacturing sector. The application of viral vectors in vaccine development against SARS-CoV-2 has witnessed remarkable growth in the fiscal year 2020. By far, the manufacturing of viral vectors for research use is impeded by a lack of production capacity to fulfill the growing market needs.
Thus, operating key stakeholders are engaged in implementing new approaches to overcome these challenges and expand production capacities. The improving ratio of clinical success to the number of clinical trials of gene and cell therapy products is a testament to the enhancing manufacturing capabilities.
Currently, several new gene therapy products are in the late stages of development and the pipeline continues to expand across the globe. The forward momentum for the advanced therapy arena is anticipated to drive investment to conduct research for the development of safe viral vectors and therapies.
Viral Vector Production (Research-use) Market Report Highlights
- Adeno-associated virus (AAV) accounted for the largest revenue share in 2020. Proven records of non-pathogenicity are one of the important key factors boosting the adoption of this segment
- Recently, usage of AAV is rising considerably across several therapeutic areas, consequently witnessing a significant boost in adoption rate throughout the forecast period
- In terms of revenue share, the downstream processing segment dominated the market in 2020 owing to highly complex polishing and purification procedures of final products
- Furthermore, the growing demand for viral vectors due to their increased adoption as therapeutics has led to an increase in the need for optimizing downstream and upstream workflows
- This is driving investment flow in both segments, resulting in a significant share of upstream processing
- Given the extensive efforts in COVID-19 vaccine development, the application of viral vectors in the vaccine development segment has witnessed tremendous growth, resulting in the largest revenue share of this segment in 2020
- The research institutes segment dominated the market in terms of revenue share in 2020. The increasing involvement of scientific communities in gene and cell therapy research has driven the revenue flow in this segment
- North America captured the maximum revenue share in 2020 with the U.S. at the forefront
- One major factor that has contributed to the larger share of this regional market is the presence of a substantial number of centers and institutes that are engaged in the R&D of advanced therapies
- Investments made by the federal bodies for the expansion of cell therapy research base in the region are anticipated to enhance the growth of the market in North America
- The key market players are engaged in collaboration with pharma companies to serve their viral-vector-based research needs pertaining to advanced therapy development
This product will be delivered within 1-3 business days.
Table of Contents
Chapter 1 Executive Summary
1.1 Viral Production Market (for Research Use Only) Outlook
1.1.1 Market Summary
1.1.1 Market Summary
Chapter 2 Research Methodology
2.1 Information Procurement
2.2 Information or Data Analysis
2.3 Market Model
2.3.1 Market Analysis, by Vector Type
2.3.1.1 By characteristics/advantage & disadvantage
2.3.1.2 By clinical trials
2.3.1.3 By manufacturing service penetration/availability
2.3.2 Market Study, By End Use
2.3.3 Regional Analysis
2.2 Information or Data Analysis
2.3 Market Model
2.3.1 Market Analysis, by Vector Type
2.3.1.1 By characteristics/advantage & disadvantage
2.3.1.2 By clinical trials
2.3.1.3 By manufacturing service penetration/availability
2.3.2 Market Study, By End Use
2.3.3 Regional Analysis
Chapter 3 Market Variables, Trends, & Scope
3.1 Market Segmentation & Scope
3.2 Market Dynamics
3.2.1 Market Drivers Analysis
3.2.1.1 Robust pipeline for gene therapies and viral vector vaccines
3.2.1.2 Technological advancements in manufacturing vectors
3.2.1.3 Highly competitive market and various strategies are undertaken by market entities
3.2.2 Market Restraint Analysis
3.2.2.1 Regulatory, scientific, and ethical challenges associated with gene therapy and viral vectors
3.2.3 Market Challenge Analysis
3.2.3.1 Production capacity challenges
3.2.3.2 Manufacturing challenges pertaining to large scale production of vectors
3.2.4 Market Opportunity Analysis
3.2.4.1 Facility expansion for cell and gene therapies
3.3 Penetration & Growth Prospect Mapping for Vector Type, 2020
3.4 Industry Analysis - Porter’s
3.5 SWOT Analysis, By Factor (Political & Legal, Economic and Technological)
3.6 Viral Vector Production Capacity & Service Mapping Analysis
3.6.1 North America: Capacity & service mapping
3.6.2 Europe: Capacity & Service Mapping
3.6.2.1 Cobra Biologics (Sweden and the U.K.)
3.6.2.2 Biovian (Finland)
3.6.2.3 Oxford Biomedica (U.K.)
3.6.2.4 Lonza Pharma & Biotech (The Netherlands)
3.6.2.5 FinVector Oy (Finland)
3.6.2.6 Fujifilm Diosynth Biotechnologies (Denmark)
3.6.2.7 Fujifilm Diosynth Biotechnologies (U.K.)
3.6.2.8 Catalent Inc. (Belgium)
3.6.2.9 Novasep (Belgium)
3.6.2.10 Exothera (Belgium)
3.6.2.11 Delphi Genetics SA (Belgium)
3.6.2.12 Yposkesi (France)
3.6.2.13 VIVEbiotech (Spain)
3.6.2.14 MolMed SpA (Italy)
3.6.2.15 Anemocyte (Italy)
3.7 COVID-19 Impact Analysis
3.2 Market Dynamics
3.2.1 Market Drivers Analysis
3.2.1.1 Robust pipeline for gene therapies and viral vector vaccines
3.2.1.2 Technological advancements in manufacturing vectors
3.2.1.3 Highly competitive market and various strategies are undertaken by market entities
3.2.2 Market Restraint Analysis
3.2.2.1 Regulatory, scientific, and ethical challenges associated with gene therapy and viral vectors
3.2.3 Market Challenge Analysis
3.2.3.1 Production capacity challenges
3.2.3.2 Manufacturing challenges pertaining to large scale production of vectors
3.2.4 Market Opportunity Analysis
3.2.4.1 Facility expansion for cell and gene therapies
3.3 Penetration & Growth Prospect Mapping for Vector Type, 2020
3.4 Industry Analysis - Porter’s
3.5 SWOT Analysis, By Factor (Political & Legal, Economic and Technological)
3.6 Viral Vector Production Capacity & Service Mapping Analysis
3.6.1 North America: Capacity & service mapping
3.6.2 Europe: Capacity & Service Mapping
3.6.2.1 Cobra Biologics (Sweden and the U.K.)
3.6.2.2 Biovian (Finland)
3.6.2.3 Oxford Biomedica (U.K.)
3.6.2.4 Lonza Pharma & Biotech (The Netherlands)
3.6.2.5 FinVector Oy (Finland)
3.6.2.6 Fujifilm Diosynth Biotechnologies (Denmark)
3.6.2.7 Fujifilm Diosynth Biotechnologies (U.K.)
3.6.2.8 Catalent Inc. (Belgium)
3.6.2.9 Novasep (Belgium)
3.6.2.10 Exothera (Belgium)
3.6.2.11 Delphi Genetics SA (Belgium)
3.6.2.12 Yposkesi (France)
3.6.2.13 VIVEbiotech (Spain)
3.6.2.14 MolMed SpA (Italy)
3.6.2.15 Anemocyte (Italy)
3.7 COVID-19 Impact Analysis
Chapter 4 Vector Type Business Analysis
4.1 Viral Vector Production (Research-use) Market: Vector Type Movement Analysis
4.2 Adenovirus
4.2.1 Adenovirus Viral Vector Production (Research-use) Market Estimates and Forecast for Adenovirus, 2017 - 2028 (USD Million)
4.3 Retrovirus
4.3.1 Retrovirus Viral Vector Production (Research-use) Market Estimates and Forecast for Retrovirus 2017 - 2028 (USD Million)
4.4 Adeno-Associated Virus (AAV)
4.4.1 Adeno-Associated Virus (AAV)Viral Vector Production (Research-use) Market Estimates and Forecast for AAV, 2017 - 2028 (USD Million)
4.5 Lentivirus
4.5.1 Lentivirus Viral Vector Production (Research-use) Market Estimates and Forecast for lentivirus, 2017 - 2028 (USD Million)
4.6 Others
4.6.1 Other Viral Vector Production (Research-use) Market Estimates and Forecast for others, 2017 - 2028 (USD Million)
4.2 Adenovirus
4.2.1 Adenovirus Viral Vector Production (Research-use) Market Estimates and Forecast for Adenovirus, 2017 - 2028 (USD Million)
4.3 Retrovirus
4.3.1 Retrovirus Viral Vector Production (Research-use) Market Estimates and Forecast for Retrovirus 2017 - 2028 (USD Million)
4.4 Adeno-Associated Virus (AAV)
4.4.1 Adeno-Associated Virus (AAV)Viral Vector Production (Research-use) Market Estimates and Forecast for AAV, 2017 - 2028 (USD Million)
4.5 Lentivirus
4.5.1 Lentivirus Viral Vector Production (Research-use) Market Estimates and Forecast for lentivirus, 2017 - 2028 (USD Million)
4.6 Others
4.6.1 Other Viral Vector Production (Research-use) Market Estimates and Forecast for others, 2017 - 2028 (USD Million)
Chapter 5 Workflow Business Analysis
5.1 Viral Vector Production (Research-use) Market: Workflow Movement Analysis
5.2 Upstream Processing
5.2.1 Global Upstream Processing Market Estimates And Forecast, 2017 - 2028 (USD Million)
5.2.2 Vector amplification and expansion
5.2.2.1 Global vector amplification and expansion market estimates and forecast, 2017 - 2028 (USD Million)
5.2.3 Vector recovery/harvesting
5.2.3.1 Global vector recovery/harvesting market estimates and forecast, 2017 - 2028 (USD Million)
5.3 Downstream Processing
5.3.1 Global Downstream Processing Market Estimates And Forecast for 2017 - 2028 (USD Million)
5.3.2 Purification
5.3.2.1 Global purification market estimates and forecast, 2017 - 2028 (USD Million)
5.3.3 Fill finish
5.3.3.1 Global fill finish market estimates and forecast, 2017 - 2028 (USD Million)
5.2 Upstream Processing
5.2.1 Global Upstream Processing Market Estimates And Forecast, 2017 - 2028 (USD Million)
5.2.2 Vector amplification and expansion
5.2.2.1 Global vector amplification and expansion market estimates and forecast, 2017 - 2028 (USD Million)
5.2.3 Vector recovery/harvesting
5.2.3.1 Global vector recovery/harvesting market estimates and forecast, 2017 - 2028 (USD Million)
5.3 Downstream Processing
5.3.1 Global Downstream Processing Market Estimates And Forecast for 2017 - 2028 (USD Million)
5.3.2 Purification
5.3.2.1 Global purification market estimates and forecast, 2017 - 2028 (USD Million)
5.3.3 Fill finish
5.3.3.1 Global fill finish market estimates and forecast, 2017 - 2028 (USD Million)
Chapter 6 Application Business Analysis
6.1 Viral Vector Production (Research-use) Market: Application Movement Analysis
6.2 Gene and Cell Therapy Development
6.2.1 Viral Vector Production (Research-use) Market Estimates And Forecast for Gene and Cell Therapy Development, 2017 - 2028 (USD Million)
6.3 Vaccine Development
6.3.1 Viral Vector Production (Research-use) Market Estimates And Forecast for Vaccine development 2017 - 2028 (USD Million)
6.4 Biopharmaceutical and Pharmaceutical Discovery
6.4.1 Viral Vector Production (Research-use) Market Estimates And Forecast for Biopharmaceutical And Pharmaceutical Discovery, 2017 - 2028 (USD Million)
6.5 Biomedical Research
6.5.1 Viral Vector Production (Research-use) Market Estimates And Forecast for Biomedical Research, 2017 - 2028 (USD Million)
6.2 Gene and Cell Therapy Development
6.2.1 Viral Vector Production (Research-use) Market Estimates And Forecast for Gene and Cell Therapy Development, 2017 - 2028 (USD Million)
6.3 Vaccine Development
6.3.1 Viral Vector Production (Research-use) Market Estimates And Forecast for Vaccine development 2017 - 2028 (USD Million)
6.4 Biopharmaceutical and Pharmaceutical Discovery
6.4.1 Viral Vector Production (Research-use) Market Estimates And Forecast for Biopharmaceutical And Pharmaceutical Discovery, 2017 - 2028 (USD Million)
6.5 Biomedical Research
6.5.1 Viral Vector Production (Research-use) Market Estimates And Forecast for Biomedical Research, 2017 - 2028 (USD Million)
Chapter 7 End-Use Business Analysis
7.1 Viral Vector Production (Research-use) Market: End-use Movement Analysis
7.2 Pharmaceutical and Biopharmaceutical Companies
7.2.1 Global Viral Vector Production (Research-use) Market Estimates and Forecast for Pharmaceutical And Biopharmaceutical Companies, 2017 - 2028 (USD Million)
7.3 Research Institutes
7.3.1 Global Viral Vector Production (Research-use) Market Estimates And Forecast for Research institutes, 2017 - 2028 (USD Million)
7.2 Pharmaceutical and Biopharmaceutical Companies
7.2.1 Global Viral Vector Production (Research-use) Market Estimates and Forecast for Pharmaceutical And Biopharmaceutical Companies, 2017 - 2028 (USD Million)
7.3 Research Institutes
7.3.1 Global Viral Vector Production (Research-use) Market Estimates And Forecast for Research institutes, 2017 - 2028 (USD Million)
Chapter 8 Regional Business Analysis
8.1 Viral Vector Production (Research-use) Market: Regional Movement Analysis
8.2 North America
8.2.1 North America Vector Production (Research-use) Market Estimates And Forecast, 2017 - 2028 (USD Million)
8.2.2 U.S.
8.2.2.1 U.S. viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.2.2.2 U.S. viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.2.2.3 U.S. viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.2.2.4 U.S. viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.2.3 Canada
8.2.3.1 Canada viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.2.3.2 Canada viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.2.3.3 Canada viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.2.3.4 Canada viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.3 Europe
8.3.1 Europe Viral Vector Production (Research-use) Market Estimates And Forecast, 2017 - 2028 (USD Million)
8.3.2 Germany
8.3.2.1 Germany viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.3.2.2 Germany viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.3.2.3 Germany viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.3.2.4 Germany viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.3.3 U.K.
8.3.3.1 U.K. viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.3.3.2 U.K. viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.3.3.3 U.K. viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.3.3.4 U.K. viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.3.4 France
8.3.4.1 France viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.3.4.2 France viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.3.4.3 France viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.3.4.4 France viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.3.5 Spain
8.3.5.1 Spain viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.3.5.2 Spain viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.3.5.3 Spain viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.3.5.4 Spain viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.3.6 Italy
8.3.6.1 Italy viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.3.6.2 Italy viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.3.6.3 Italy viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.3.6.4 Italy viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.4 Asia Pacific
8.4.1 Viral Vector Production (Research-use) Market Estimates And Forecast, 2017 - 2028 (USD Million)
8.4.2 China
8.4.2.1 China viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.4.2.2 China viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.4.2.3 China viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.4.2.4 China viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.4.3 Japan
8.4.3.1 Japan viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.4.3.2 Japan viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.4.3.3 Japan viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.4.3.4 Japan viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.4.4 India
8.4.4.1 India viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.4.4.2 India viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.4.4.3 India viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.4.4.4 India viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.4.5 South Korea
8.4.5.1 South Korea viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.4.5.2 South Korea viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.4.5.3 South Korea viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.4.5.4 South Korea viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.4.6 Australia
8.4.6.1 Australia viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.4.6.2 Australia viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.4.6.3 Australia viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.4.6.4 Australia viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.5 Latin America
8.5.1 Latin America Viral Vector Production (Research-use) Market Estimates And Forecast, 2017 - 2028 (USD Million)
8.5.2 Brazil
8.5.2.1 Brazil viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.5.2.2 Brazil viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.5.2.3 Brazil viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.5.2.4 Brazil viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.5.3 Mexico
8.5.3.1 Mexico viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.5.3.2 Mexico viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.5.3.3 Mexico viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.5.3.4 Mexico viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.6 MEA
8.6.1 MEA Viral Vector Production (Research-use) Market Estimates And Forecast, 2017 - 2028 (USD Million)
8.6.2 South Africa
8.6.2.1 South Africa viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.6.2.2 South Africa viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.6.2.3 South Africa viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.6.2.4 South Africa viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.6.3 Saudi Arabia
8.6.3.1 Saudi Arabia viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.6.3.2 Saudi Arabia viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.6.3.3 Saudi Arabia viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.6.3.4 Saudi Arabia viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.2 North America
8.2.1 North America Vector Production (Research-use) Market Estimates And Forecast, 2017 - 2028 (USD Million)
8.2.2 U.S.
8.2.2.1 U.S. viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.2.2.2 U.S. viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.2.2.3 U.S. viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.2.2.4 U.S. viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.2.3 Canada
8.2.3.1 Canada viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.2.3.2 Canada viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.2.3.3 Canada viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.2.3.4 Canada viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.3 Europe
8.3.1 Europe Viral Vector Production (Research-use) Market Estimates And Forecast, 2017 - 2028 (USD Million)
8.3.2 Germany
8.3.2.1 Germany viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.3.2.2 Germany viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.3.2.3 Germany viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.3.2.4 Germany viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.3.3 U.K.
8.3.3.1 U.K. viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.3.3.2 U.K. viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.3.3.3 U.K. viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.3.3.4 U.K. viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.3.4 France
8.3.4.1 France viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.3.4.2 France viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.3.4.3 France viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.3.4.4 France viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.3.5 Spain
8.3.5.1 Spain viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.3.5.2 Spain viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.3.5.3 Spain viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.3.5.4 Spain viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.3.6 Italy
8.3.6.1 Italy viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.3.6.2 Italy viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.3.6.3 Italy viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.3.6.4 Italy viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.4 Asia Pacific
8.4.1 Viral Vector Production (Research-use) Market Estimates And Forecast, 2017 - 2028 (USD Million)
8.4.2 China
8.4.2.1 China viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.4.2.2 China viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.4.2.3 China viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.4.2.4 China viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.4.3 Japan
8.4.3.1 Japan viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.4.3.2 Japan viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.4.3.3 Japan viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.4.3.4 Japan viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.4.4 India
8.4.4.1 India viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.4.4.2 India viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.4.4.3 India viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.4.4.4 India viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.4.5 South Korea
8.4.5.1 South Korea viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.4.5.2 South Korea viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.4.5.3 South Korea viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.4.5.4 South Korea viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.4.6 Australia
8.4.6.1 Australia viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.4.6.2 Australia viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.4.6.3 Australia viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.4.6.4 Australia viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.5 Latin America
8.5.1 Latin America Viral Vector Production (Research-use) Market Estimates And Forecast, 2017 - 2028 (USD Million)
8.5.2 Brazil
8.5.2.1 Brazil viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.5.2.2 Brazil viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.5.2.3 Brazil viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.5.2.4 Brazil viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.5.3 Mexico
8.5.3.1 Mexico viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.5.3.2 Mexico viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.5.3.3 Mexico viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.5.3.4 Mexico viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.6 MEA
8.6.1 MEA Viral Vector Production (Research-use) Market Estimates And Forecast, 2017 - 2028 (USD Million)
8.6.2 South Africa
8.6.2.1 South Africa viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.6.2.2 South Africa viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.6.2.3 South Africa viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.6.2.4 South Africa viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
8.6.3 Saudi Arabia
8.6.3.1 Saudi Arabia viral vector production (research-use) market estimates and forecast, by vector type, 2017 - 2028 (USD Million)
8.6.3.2 Saudi Arabia viral vector production (research-use) market estimates and forecast, by workflow, 2017 - 2028 (USD Million)
8.6.3.3 Saudi Arabia viral vector production (research-use) market estimates and forecast, by application, 2017 - 2028 (USD Million)
8.6.3.4 Saudi Arabia viral vector production (research-use) market estimates and forecast, by end use, 2017 - 2028 (USD Million)
Chapter 9 Competitive Analysis
9.1 Recent Developments & Impact Analysis, by Key Market Participants
9.2 Company/Competition Categorization
9.3 Manufacturer’s Landscape
9.3.1 Distribution by Location of Manufacturing Facility
9.3.2 CDMOs Operating in China
9.3.3 Distribution by Scale of Production
9.3.4 Distribution by Location Of Manufacturing Facility, Type Of Organization, And Purpose Of Production
9.4 Vendor Landscape
9.4.1 List of Key Technology Supplier
9.4.2 List of Raw Material/Technology Distributors
9.4.2.1 Region-wise Bioprocessing Technology Distributors:
9.5 List of Companies with Portfolio Comprising Viral Vector-based Therapeutic Candidates.
9.6 List of Companies with Service Portfolio offering Viral Vector Manufacturing Services.
9.7 Key Initiatives & Strategic Alliances Analysis
9.7.1 Merger & Acquisition Deals
9.7.2 Collaborations & Partnerships
9.7.3 Business Expansion
9.7.4 Technology Collaborations
9.8 Public Companies Analysis
9.8.1 Industry Players
9.8.1.1 Takara Bio Inc.
9.8.1.2 Fujifilm Diosynth Biotechnologies
9.8.1.3 Batavia Biosciences B.V.
9.8.2 Non-Industry Players
9.8.3 Synergy Analysis: Major Deals & Strategic Alliances
9.9 Private Companies
9.9.1 List of key emerging companies
9.9.2 Market Participation Categorization (Market Operations & Weakness)
9.9.3 Strategy Framework
9.10 Company Profiles: Contract Manufacturing Organizations (CMOs)
9.10.1 Merck kgaa
9.10.1.1 Company overview
9.10.1.2 Sigma-aldrich Inc.
9.10.1.3 Financial Performance
9.10.1.4 Product benchmarking
9.10.1.5 SWOT Analysis
9.10.1.6 Operational Capacity
9.10.1.7 strategic initiatives
9.10.2 Lonza
9.10.2.1 Company overview
9.10.2.2 Financial Performance
9.10.2.3 Product benchmarking
9.10.2.4 SWOT Analysis
9.10.2.5 Operational Capacity
9.10.2.6 Strategic initiatives
9.10.3 FUJIFILM Diosynth Biotechnologies U.S.A., Inc.
9.10.3.1 Company overview
9.10.3.2 Financial Performance
9.10.3.3 Product benchmarking
9.10.3.4 SWOT Analysis
9.10.3.5 Operational Capacity
9.10.3.6 Strategic initiatives
9.10.4 Cobra Biologics Ltd.
9.10.4.1 Company overview
9.10.4.2 Product benchmarking
9.10.4.3 SWOT Analysis
9.10.4.4 Operational capacity
9.10.4.5 strategic initiatives
9.10.5 Thermofisher Scientific Inc.
9.10.5.1 Company overview
9.10.5.2 Financial Performance
9.10.5.3 Product benchmarking
9.10.5.4 SWOT analysis
9.10.5.5 Operational capacity
9.10.5.6 Strategic initiatives
9.10.6 Waisman Biomanufacturing
9.10.6.1 Company overview
9.10.6.2 Product benchmarking
9.10.6.3 Swot analysis
9.10.6.4 Operational capacity
9.10.6.5 Strategic initiatives
9.10.7 genezen laboratories
9.10.7.1 Company overview
9.10.7.2 Product benchmarking
9.10.7.3 SWOT Analysis
9.10.7.4 Operational capacity
9.10.8 YPOSKESI
9.10.8.1 Company overview
9.10.8.2 Product benchmarking
9.10.8.3 SWOT Analysis
9.10.8.4 Operational capacity
9.10.8.5 Strategic initiatives
9.10.9 Advanced BioScience Laboratories, Inc. (ABL inc.)
9.10.9.1 Company overview
9.10.9.2 Financial Performance
9.10.9.3 Product benchmarking
9.10.9.4 SWOT Analysis
9.10.9.5 Operational capacity
9.10.9.6 Strategic initiatives
9.10.10 Novasep Holding s.a.s.
9.10.10.1 Company overview
9.10.10.2 Product benchmarking
9.10.10.3 SWOT Analysis
9.10.10.4 Operational Capacity
9.10.10.5 Strategic initiatives
9.10.11 Orgenesis Biotech Israel Ltd (formerly ATVIO Biotech Ltd.)
9.10.11.1 Company overview
9.10.11.2 Financial Performance
9.10.11.3 Product benchmarking
9.10.11.4 SWOT Analysis
9.10.11.5 Operational Capacity
9.10.11.6 Strategic initiatives
9.10.12 Vigene biosciences Inc.
9.10.12.1 Company overview
9.10.12.2 Product benchmarking
9.10.12.3 SWOT Analysis
9.10.12.4 Operational capacity
9.10.12.5 Strategic initiatives
9.10.13 General electric company (GE healthcare)
9.10.13.1 Company overview
9.10.13.2 Financial Performance
9.10.13.3 Product benchmarking
9.10.13.4 SWOT Analysis
9.10.13.5 Operational capacity
9.10.13.6 Strategic initiatives
9.10.14 CEVEC. pharmaceuticals gmbh
9.10.14.1 Company overview
9.10.14.2 Product benchmarking
9.10.14.3 SWOT Analysis
9.10.14.4 Operational capacity
9.10.14.5 Strategic initiatives
9.10.15 Batavia Biosciences B.v.
9.10.15.1 Company overview
9.10.15.2 Product benchmarking
9.10.15.3 SWOT Analysis
9.10.15.4 Operational capacity
9.10.15.5 Strategic initiatives
9.10.16 Biovion oy
9.10.16.1 Company overview
9.10.16.2 Product benchmarking
9.10.16.3 SWOT Analysis
9.10.16.4 Operational capacity
9.10.16.5 Strategic initiatives
9.10.17 Wuxi AppTec Co., Ltd.
9.10.17.1 Company overview
9.10.17.2 Financial Performance
9.10.17.3 Product benchmarking
9.10.17.4 SWOT Analysis
9.10.17.5 Operational capacity
9.10.17.6 Strategic initiatives
9.10.18 VGXI, Inc.
9.10.18.1 Company overview
9.10.18.2 Product benchmarking
9.10.18.3 SWOT Analysis
9.10.18.4 Operational capacity
9.10.18.5 Strategic initiatives5
9.10.19 Catalent Inc.
9.10.19.1 Company overview
9.10.19.2 Paragon Bioservices Inc.
9.10.19.3 Financial performance
9.10.19.4 Product benchmarking
9.10.19.5 SWOT Analysis
9.10.19.6 Operational capacity
9.10.19.7 Strategic initiatives
9.10.20 Miltenyi Biotec gmbh
9.10.20.1 Company overview
9.10.20.2 Lentigen technology Inc.
9.10.20.3 Product benchmarking
9.10.20.4 SWOT Analysis
9.10.20.5 Operational capacity
9.10.20.6 Strategic initiatives
9.10.21 Sirion biotech gmbh
9.10.21.1 Company overview
9.10.21.2 Product benchmarking
9.10.21.3 SWOT Analysis
9.10.21.4 Operational capacity
9.10.21.5 Strategic initiatives
9.10.22 Virovek incorporation
9.10.22.1 Company overview
9.10.22.2 Product benchmarking
9.10.22.3 SWOT Analysis
9.10.22.4 Operational capacity
9.10.22.5 Strategic initiatives
9.10.23 BioNTech IMFS GmbH
9.10.23.1 Company overview
9.10.23.2 Product benchmarking
9.10.23.3 SWOT Analysis
9.10.23.4 Operational capacity
9.10.24 VIVEbiotech s.l.
9.10.24.1 Company overview
9.10.24.2 Financial Performance
9.10.24.3 Product benchmarking
9.10.24.4 SWOT Analysis
9.10.24.5 Operational capacity
9.10.24.6 Strategic initiatives
9.10.25 Creative biogene
9.10.25.1 Company overview
9.10.25.2 Product benchmarking
9.10.25.3 SWOT Analysis
9.10.26 Vibalogics GmbH
9.10.26.1 Company overview
9.10.26.2 Product benchmarking
9.10.26.3 SWOT Analysis
9.10.26.4 Operational capacity
9.10.26.5 Strategic initiatives
9.10.27 Takara Bio Inc.
9.10.27.1 Company overview
9.10.27.2 Financial Performance
9.10.27.3 Product benchmarking
9.10.27.4 SWOT Analysis
9.10.27.5 Operational capacity
9.10.27.6 Strategic initiatives
9.11 Company Profiles: In-house Manufacturers
9.11.1 Cell and Gene Therapy Catapult
9.11.1.1 Company overview
9.11.1.2 Product benchmarking
9.11.1.3 SWOT Analysis
9.11.1.4 Operational capacity
9.11.1.5 Strategic initiatives
9.11.2 Bluebird Bio Inc.
9.11.2.1 Company overview
9.11.2.2 Financial Performance
9.11.2.3 Product benchmarking
9.11.2.4 SWOT Analysis
9.11.2.5 Operational capacity
9.11.2.6 Strategic initiatives
9.11.3 Addgene Inc.
9.11.3.1 Company overview
9.11.3.2 Product benchmarking
9.11.3.3 SWOT Analysis
9.11.3.4 Operational capacity
9.11.3.5 Strategic initiatives
9.11.4 Aldevron LLC.
9.11.4.1 Company overview
9.11.4.2 Product benchmarking
9.11.4.3 SWOT Analysis
9.11.4.4 Operational capacity
9.11.4.5 Strategic initiatives
9.11.5 Astellas Pharma, Inc.
9.11.5.1 Company overview
9.11.5.2 Audentes Therapeutics
9.11.5.3 Financial performance (Astellas Pharma Inc.)
9.11.5.4 Product benchmarking
9.11.5.5 SWOT Analysis
9.11.5.6 Operational capacity
9.11.5.7 Strategic initiatives
9.11.6 BioMarin Pharmaceutical, Inc.
9.11.6.1 Company overview
9.11.6.2 Financial Performance
9.11.6.3 Product benchmarking
9.11.6.4 SWOT Analysis
9.11.6.5 Operational capacity
9.11.6.6 Strategic initiatives
9.11.7 RegenxBio, Inc.
9.11.7.1 Company overview
9.11.7.2 Financial Performance
9.11.7.3 Product benchmarking
9.11.7.4 SWOT Analysis
9.11.7.5 Operational capacity
9.11.7.6 SWOT Analysis
9.2 Company/Competition Categorization
9.3 Manufacturer’s Landscape
9.3.1 Distribution by Location of Manufacturing Facility
9.3.2 CDMOs Operating in China
9.3.3 Distribution by Scale of Production
9.3.4 Distribution by Location Of Manufacturing Facility, Type Of Organization, And Purpose Of Production
9.4 Vendor Landscape
9.4.1 List of Key Technology Supplier
9.4.2 List of Raw Material/Technology Distributors
9.4.2.1 Region-wise Bioprocessing Technology Distributors:
9.5 List of Companies with Portfolio Comprising Viral Vector-based Therapeutic Candidates.
9.6 List of Companies with Service Portfolio offering Viral Vector Manufacturing Services.
9.7 Key Initiatives & Strategic Alliances Analysis
9.7.1 Merger & Acquisition Deals
9.7.2 Collaborations & Partnerships
9.7.3 Business Expansion
9.7.4 Technology Collaborations
9.8 Public Companies Analysis
9.8.1 Industry Players
9.8.1.1 Takara Bio Inc.
9.8.1.2 Fujifilm Diosynth Biotechnologies
9.8.1.3 Batavia Biosciences B.V.
9.8.2 Non-Industry Players
9.8.3 Synergy Analysis: Major Deals & Strategic Alliances
9.9 Private Companies
9.9.1 List of key emerging companies
9.9.2 Market Participation Categorization (Market Operations & Weakness)
9.9.3 Strategy Framework
9.10 Company Profiles: Contract Manufacturing Organizations (CMOs)
9.10.1 Merck kgaa
9.10.1.1 Company overview
9.10.1.2 Sigma-aldrich Inc.
9.10.1.3 Financial Performance
9.10.1.4 Product benchmarking
9.10.1.5 SWOT Analysis
9.10.1.6 Operational Capacity
9.10.1.7 strategic initiatives
9.10.2 Lonza
9.10.2.1 Company overview
9.10.2.2 Financial Performance
9.10.2.3 Product benchmarking
9.10.2.4 SWOT Analysis
9.10.2.5 Operational Capacity
9.10.2.6 Strategic initiatives
9.10.3 FUJIFILM Diosynth Biotechnologies U.S.A., Inc.
9.10.3.1 Company overview
9.10.3.2 Financial Performance
9.10.3.3 Product benchmarking
9.10.3.4 SWOT Analysis
9.10.3.5 Operational Capacity
9.10.3.6 Strategic initiatives
9.10.4 Cobra Biologics Ltd.
9.10.4.1 Company overview
9.10.4.2 Product benchmarking
9.10.4.3 SWOT Analysis
9.10.4.4 Operational capacity
9.10.4.5 strategic initiatives
9.10.5 Thermofisher Scientific Inc.
9.10.5.1 Company overview
9.10.5.2 Financial Performance
9.10.5.3 Product benchmarking
9.10.5.4 SWOT analysis
9.10.5.5 Operational capacity
9.10.5.6 Strategic initiatives
9.10.6 Waisman Biomanufacturing
9.10.6.1 Company overview
9.10.6.2 Product benchmarking
9.10.6.3 Swot analysis
9.10.6.4 Operational capacity
9.10.6.5 Strategic initiatives
9.10.7 genezen laboratories
9.10.7.1 Company overview
9.10.7.2 Product benchmarking
9.10.7.3 SWOT Analysis
9.10.7.4 Operational capacity
9.10.8 YPOSKESI
9.10.8.1 Company overview
9.10.8.2 Product benchmarking
9.10.8.3 SWOT Analysis
9.10.8.4 Operational capacity
9.10.8.5 Strategic initiatives
9.10.9 Advanced BioScience Laboratories, Inc. (ABL inc.)
9.10.9.1 Company overview
9.10.9.2 Financial Performance
9.10.9.3 Product benchmarking
9.10.9.4 SWOT Analysis
9.10.9.5 Operational capacity
9.10.9.6 Strategic initiatives
9.10.10 Novasep Holding s.a.s.
9.10.10.1 Company overview
9.10.10.2 Product benchmarking
9.10.10.3 SWOT Analysis
9.10.10.4 Operational Capacity
9.10.10.5 Strategic initiatives
9.10.11 Orgenesis Biotech Israel Ltd (formerly ATVIO Biotech Ltd.)
9.10.11.1 Company overview
9.10.11.2 Financial Performance
9.10.11.3 Product benchmarking
9.10.11.4 SWOT Analysis
9.10.11.5 Operational Capacity
9.10.11.6 Strategic initiatives
9.10.12 Vigene biosciences Inc.
9.10.12.1 Company overview
9.10.12.2 Product benchmarking
9.10.12.3 SWOT Analysis
9.10.12.4 Operational capacity
9.10.12.5 Strategic initiatives
9.10.13 General electric company (GE healthcare)
9.10.13.1 Company overview
9.10.13.2 Financial Performance
9.10.13.3 Product benchmarking
9.10.13.4 SWOT Analysis
9.10.13.5 Operational capacity
9.10.13.6 Strategic initiatives
9.10.14 CEVEC. pharmaceuticals gmbh
9.10.14.1 Company overview
9.10.14.2 Product benchmarking
9.10.14.3 SWOT Analysis
9.10.14.4 Operational capacity
9.10.14.5 Strategic initiatives
9.10.15 Batavia Biosciences B.v.
9.10.15.1 Company overview
9.10.15.2 Product benchmarking
9.10.15.3 SWOT Analysis
9.10.15.4 Operational capacity
9.10.15.5 Strategic initiatives
9.10.16 Biovion oy
9.10.16.1 Company overview
9.10.16.2 Product benchmarking
9.10.16.3 SWOT Analysis
9.10.16.4 Operational capacity
9.10.16.5 Strategic initiatives
9.10.17 Wuxi AppTec Co., Ltd.
9.10.17.1 Company overview
9.10.17.2 Financial Performance
9.10.17.3 Product benchmarking
9.10.17.4 SWOT Analysis
9.10.17.5 Operational capacity
9.10.17.6 Strategic initiatives
9.10.18 VGXI, Inc.
9.10.18.1 Company overview
9.10.18.2 Product benchmarking
9.10.18.3 SWOT Analysis
9.10.18.4 Operational capacity
9.10.18.5 Strategic initiatives5
9.10.19 Catalent Inc.
9.10.19.1 Company overview
9.10.19.2 Paragon Bioservices Inc.
9.10.19.3 Financial performance
9.10.19.4 Product benchmarking
9.10.19.5 SWOT Analysis
9.10.19.6 Operational capacity
9.10.19.7 Strategic initiatives
9.10.20 Miltenyi Biotec gmbh
9.10.20.1 Company overview
9.10.20.2 Lentigen technology Inc.
9.10.20.3 Product benchmarking
9.10.20.4 SWOT Analysis
9.10.20.5 Operational capacity
9.10.20.6 Strategic initiatives
9.10.21 Sirion biotech gmbh
9.10.21.1 Company overview
9.10.21.2 Product benchmarking
9.10.21.3 SWOT Analysis
9.10.21.4 Operational capacity
9.10.21.5 Strategic initiatives
9.10.22 Virovek incorporation
9.10.22.1 Company overview
9.10.22.2 Product benchmarking
9.10.22.3 SWOT Analysis
9.10.22.4 Operational capacity
9.10.22.5 Strategic initiatives
9.10.23 BioNTech IMFS GmbH
9.10.23.1 Company overview
9.10.23.2 Product benchmarking
9.10.23.3 SWOT Analysis
9.10.23.4 Operational capacity
9.10.24 VIVEbiotech s.l.
9.10.24.1 Company overview
9.10.24.2 Financial Performance
9.10.24.3 Product benchmarking
9.10.24.4 SWOT Analysis
9.10.24.5 Operational capacity
9.10.24.6 Strategic initiatives
9.10.25 Creative biogene
9.10.25.1 Company overview
9.10.25.2 Product benchmarking
9.10.25.3 SWOT Analysis
9.10.26 Vibalogics GmbH
9.10.26.1 Company overview
9.10.26.2 Product benchmarking
9.10.26.3 SWOT Analysis
9.10.26.4 Operational capacity
9.10.26.5 Strategic initiatives
9.10.27 Takara Bio Inc.
9.10.27.1 Company overview
9.10.27.2 Financial Performance
9.10.27.3 Product benchmarking
9.10.27.4 SWOT Analysis
9.10.27.5 Operational capacity
9.10.27.6 Strategic initiatives
9.11 Company Profiles: In-house Manufacturers
9.11.1 Cell and Gene Therapy Catapult
9.11.1.1 Company overview
9.11.1.2 Product benchmarking
9.11.1.3 SWOT Analysis
9.11.1.4 Operational capacity
9.11.1.5 Strategic initiatives
9.11.2 Bluebird Bio Inc.
9.11.2.1 Company overview
9.11.2.2 Financial Performance
9.11.2.3 Product benchmarking
9.11.2.4 SWOT Analysis
9.11.2.5 Operational capacity
9.11.2.6 Strategic initiatives
9.11.3 Addgene Inc.
9.11.3.1 Company overview
9.11.3.2 Product benchmarking
9.11.3.3 SWOT Analysis
9.11.3.4 Operational capacity
9.11.3.5 Strategic initiatives
9.11.4 Aldevron LLC.
9.11.4.1 Company overview
9.11.4.2 Product benchmarking
9.11.4.3 SWOT Analysis
9.11.4.4 Operational capacity
9.11.4.5 Strategic initiatives
9.11.5 Astellas Pharma, Inc.
9.11.5.1 Company overview
9.11.5.2 Audentes Therapeutics
9.11.5.3 Financial performance (Astellas Pharma Inc.)
9.11.5.4 Product benchmarking
9.11.5.5 SWOT Analysis
9.11.5.6 Operational capacity
9.11.5.7 Strategic initiatives
9.11.6 BioMarin Pharmaceutical, Inc.
9.11.6.1 Company overview
9.11.6.2 Financial Performance
9.11.6.3 Product benchmarking
9.11.6.4 SWOT Analysis
9.11.6.5 Operational capacity
9.11.6.6 Strategic initiatives
9.11.7 RegenxBio, Inc.
9.11.7.1 Company overview
9.11.7.2 Financial Performance
9.11.7.3 Product benchmarking
9.11.7.4 SWOT Analysis
9.11.7.5 Operational capacity
9.11.7.6 SWOT Analysis
List of Tables
Table 1 Viral vector characteristic analysis
Table 2 Viral vector-based COVID-19 vaccine candidates
Table 3 Operating CMOs/CDMOs for advanced therapy manufacturing in EU
Table 4 Most-used 2D devices used for LV production
Table 5 Fixed-bed bioreactors optimized for high-scale LV production
Table 6 Human clinical trials in progress with viral vectored vaccines
Table 7 List of key technology suppliers for downstream processing
Table 8 List of key technology suppliers for upstream processing
Table 9 Distributors list for Sartorius for key countries
Table 10 Distributors list for Cytiva for key countries
Table 11 List of key emerging companies
Table 12 Contract Manufacturing Organizations (CMOs) offering viral & non-viral vector manufacturing services
Table 13 Biozilla, LLC’s plasmid purification cost
Table 14 AAV production: Pricing analysis
Table 15 AAV packaging, cloning, & titration: Pricing analysis
Table 16 Lentivirus production: Pricing analysis
Table 17 Lentivirus packaging, titration, & others: Pricing analysis
Table 18 Adenovirus production: Pricing analysis
Table 19 Adenovirus packaging, titration, & others: Pricing analysis
Table 20 Retrovirus production: Pricing analysis
Table 21 Plasmid production: Pricing analysis
Table 22 Plasmid DNA purification: Pricing analysis
Table 23 Eurofins Genomics LLC’s gene synthesis cost analysis
Table 24 GenScript’s gene synthesis cost analysis (GenPlus Economy Gene Synthesis)
Table 25 Eurofins Genomics LLC’s gene fragments cost analysis
Table 26 North America viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 27 North America viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 28 North America viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 29 North America viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 30 North America viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 31 North America viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 32 U.S. viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 33 U.S. viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 34 U.S. viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 35 U.S. viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 36 U.S. viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 37 U.S. viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 38 Canada viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 39 Canada viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 40 Canada viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 41 Canada viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 42 Canada viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 43 Canada viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 44 Europe viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 45 Europe viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 46 Europe viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 47 Europe viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 48 Europe viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 49 Europe viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 50 Germany viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 51 Germany viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 52 Germany viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 53 Germany viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 54 Germany viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 55 Germany viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 56 U.K. viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 57 U.K. viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 58 U.K. viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 59 U.K. viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 60 U.K. viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 61 U.K. viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 62 France viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 63 France viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 64 France viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 65 France viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 66 France viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 67 France viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 68 Spain viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 69 Spain viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 70 Spain viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 71 Spain viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 72 Spain viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 73 Spain viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 74 Italy viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 75 Italy viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 76 Italy viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 77 Italy viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 78 Italy viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 79 Italy viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 80 Asia Pacific viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 81 Asia Pacific viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 82 Asia Pacific viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 83 Asia Pacific viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 84 Asia Pacific viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 85 Asia Pacific viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 86 China viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 87 China viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 88 China viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 89 China viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 90 China viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 91 China viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 92 Japan viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 93 Japan viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 94 Japan viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 95 Japan viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 96 Japan viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 97 Japan viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 98 India viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 99 India viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 100 India viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 101 India viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 102 India viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 103 India viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 104 South Korea viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 105 South Korea viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 106 South Korea viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 107 South Korea viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 108 South Korea viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 109 South Korea viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 110 Australia viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 111 Australia viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 112 Australia viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 113 Australia viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 114 Australia viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 115 Australia viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 116 Latin America viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 117 Latin America viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 118 Latin America viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 119 Latin America viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 120 Latin America viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 121 Latin America viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 122 Brazil viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 123 Brazil viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 124 Brazil viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 125 Brazil viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 126 Brazil viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 127 Brazil viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 128 Mexico viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 129 Mexico viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 130 Mexico viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 131 Mexico viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 132 Mexico viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 133 Mexico viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 134 MEA viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 135 MEA viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 136 MEA viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 137 MEA viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 138 MEA viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 139 MEA viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 140 South Africa viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 141 South Africa viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 142 South Africa viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 143 South Africa viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 144 South Africa viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 145 South Africa viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 146 Saudi Arabia viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 147 Saudi Arabia viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 148 Saudi Arabia viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 149 Saudi Arabia viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 150 Saudi Arabia viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 151 Saudi Arabia viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 2 Viral vector-based COVID-19 vaccine candidates
Table 3 Operating CMOs/CDMOs for advanced therapy manufacturing in EU
Table 4 Most-used 2D devices used for LV production
Table 5 Fixed-bed bioreactors optimized for high-scale LV production
Table 6 Human clinical trials in progress with viral vectored vaccines
Table 7 List of key technology suppliers for downstream processing
Table 8 List of key technology suppliers for upstream processing
Table 9 Distributors list for Sartorius for key countries
Table 10 Distributors list for Cytiva for key countries
Table 11 List of key emerging companies
Table 12 Contract Manufacturing Organizations (CMOs) offering viral & non-viral vector manufacturing services
Table 13 Biozilla, LLC’s plasmid purification cost
Table 14 AAV production: Pricing analysis
Table 15 AAV packaging, cloning, & titration: Pricing analysis
Table 16 Lentivirus production: Pricing analysis
Table 17 Lentivirus packaging, titration, & others: Pricing analysis
Table 18 Adenovirus production: Pricing analysis
Table 19 Adenovirus packaging, titration, & others: Pricing analysis
Table 20 Retrovirus production: Pricing analysis
Table 21 Plasmid production: Pricing analysis
Table 22 Plasmid DNA purification: Pricing analysis
Table 23 Eurofins Genomics LLC’s gene synthesis cost analysis
Table 24 GenScript’s gene synthesis cost analysis (GenPlus Economy Gene Synthesis)
Table 25 Eurofins Genomics LLC’s gene fragments cost analysis
Table 26 North America viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 27 North America viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 28 North America viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 29 North America viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 30 North America viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 31 North America viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 32 U.S. viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 33 U.S. viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 34 U.S. viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 35 U.S. viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 36 U.S. viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 37 U.S. viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 38 Canada viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 39 Canada viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 40 Canada viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 41 Canada viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 42 Canada viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 43 Canada viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 44 Europe viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 45 Europe viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 46 Europe viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 47 Europe viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 48 Europe viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 49 Europe viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 50 Germany viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 51 Germany viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 52 Germany viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 53 Germany viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 54 Germany viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 55 Germany viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 56 U.K. viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 57 U.K. viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 58 U.K. viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 59 U.K. viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 60 U.K. viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 61 U.K. viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 62 France viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 63 France viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 64 France viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 65 France viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 66 France viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 67 France viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 68 Spain viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 69 Spain viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 70 Spain viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 71 Spain viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 72 Spain viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 73 Spain viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 74 Italy viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 75 Italy viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 76 Italy viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 77 Italy viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 78 Italy viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 79 Italy viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 80 Asia Pacific viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 81 Asia Pacific viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 82 Asia Pacific viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 83 Asia Pacific viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 84 Asia Pacific viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 85 Asia Pacific viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 86 China viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 87 China viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 88 China viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 89 China viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 90 China viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 91 China viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 92 Japan viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 93 Japan viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 94 Japan viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 95 Japan viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 96 Japan viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 97 Japan viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 98 India viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 99 India viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 100 India viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 101 India viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 102 India viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 103 India viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 104 South Korea viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 105 South Korea viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 106 South Korea viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 107 South Korea viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 108 South Korea viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 109 South Korea viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 110 Australia viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 111 Australia viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 112 Australia viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 113 Australia viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 114 Australia viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 115 Australia viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 116 Latin America viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 117 Latin America viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 118 Latin America viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 119 Latin America viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 120 Latin America viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 121 Latin America viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 122 Brazil viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 123 Brazil viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 124 Brazil viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 125 Brazil viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 126 Brazil viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 127 Brazil viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 128 Mexico viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 129 Mexico viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 130 Mexico viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 131 Mexico viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 132 Mexico viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 133 Mexico viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 134 MEA viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 135 MEA viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 136 MEA viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 137 MEA viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 138 MEA viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 139 MEA viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 140 South Africa viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 141 South Africa viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 142 South Africa viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 143 South Africa viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 144 South Africa viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 145 South Africa viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
Table 146 Saudi Arabia viral vector production (research use) market estimates and forecasts, by vector type, 2017 - 2028 (USD Million)
Table 147 Saudi Arabia viral vector production (research use) market estimates and forecasts, by workflow, 2017 - 2028 (USD Million)
Table 148 Saudi Arabia viral vector production (research use) market estimates and forecasts, by upstream processing, 2017 - 2028 (USD Million)
Table 149 Saudi Arabia viral vector production (research use) market estimates and forecasts, by downstream processing, 2017 - 2028 (USD Million)
Table 150 Saudi Arabia viral vector production (research use) market estimates and forecasts, by application, 2017 - 2028 (USD Million)
Table 151 Saudi Arabia viral vector production (research use) market estimates and forecasts, by end use, 2017 - 2028 (USD Million)
List of Figures
Fig. 1 Market snapshot, 2020 (USD Million)
Fig. 2 Market research process
Fig. 3 Information procurement
Fig. 4 Primary research process
Fig. 5 Primary research validation
Fig. 6 Market research approaches
Fig. 7 Value-chain-based sizing & forecasting
Fig. 8 QFD modeling
Fig. 9 Market formulation and validation
Fig. 10 Vector type characteristic analysis
Fig. 11 QFD model assessment for vector type
Fig. 12 Vector type characteristic analysis
Fig. 13 Lentiviral vectors versus AAV vectors
Fig. 14 Global number of AAV- and lentivirus-based clinical trials
Fig. 15 Gene therapy approvals versus clinical trials
Fig. 16 Some major industry sponsors of CAR T cell clinical trials, as of September 2019
Fig. 17 Distribution of the sponsors of advanced therapies clinical trials, 2016
Fig. 18 Distribution of noncommercial sponsors of ATMP clinical trials, 2016
Fig. 19 Gene therapy approvals versus clinical trials
Fig. 20 Penetration by end use settings
Fig. 21 Regional analysis for rare disease genetic testing market
Fig. 22 U.K. cell and gene therapy manufacturing capacity, 2016 to 2019
Fig. 23 Number of gene therapy sites across gene therapy and multifunctional facilities in the U.K., 2019
Fig. 24 Market trends & outlook
Fig. 25 Market segmentation & scope
Fig. 26 Market driver relevance analysis (Current & future impact)
Fig. 27 Gene therapy clinical trials
Fig. 28 Viral vectors used in clinical trials
Fig. 29 Key strategies by market players to drive success in the gene therapy arena
Fig. 30 Recent and upcoming approved gene transfer-mediated programs
Fig. 31 Market restraint relevance analysis (Current & future impact)
Fig. 32 Various ethical questions raised by gene therapy
Fig. 33 Major challenges in large-scale vector manufacturing
Fig. 34 Penetration & growth prospect mapping for vector type, 2020
Fig. 35 Porter’s Five Forces Analysis
Fig. 36 SWOT analysis, by factor (Political & Legal, Economic and Technological)
Fig. 37 Viral vector production (research use) market: Vector type outlook and key takeaways
Fig. 38 Viral vector production (research use) market: Vector type movement analysis
Fig. 39 Viral vector production (research use) market estimates and forecast for Adenovirus 2017 - 2028 (USD Million)
Fig. 40 Viral vector production (research use) market estimates and forecast for a retrovirus, 2017 - 2028 (USD Million)
Fig. 41 Viral vector production (research use) market estimates and forecast for AAV, 2017 - 2028 (USD Million)
Fig. 42 Viral vector production (research use) market estimates and forecast for Lentivirus, 2017 - 2028 (USD Million)
Fig. 43 Viral vector production (research use) market estimates and forecast for Others, 2017 - 2028 (USD Million)
Fig. 44 Viral vector production (research use) market: Workflow outlook and key takeaways
Fig. 45 Viral vector production (research use) market: Workflow movement analysis
Fig. 46 Global upstream processing market estimates and forecast 2017 - 2028 (USD Million)
Fig. 47 Global vector amplification and expansion market estimates and forecast 2017 - 2028 (USD Million)
Fig. 48 Global vector recovery/harvesting market estimates and forecast 2017 - 2028 (USD Million)
Fig. 49 Global downstream processing market estimates and forecast, for 2017 - 2028 (USD Million)
Fig. 50 Global purification market estimates and forecast 2017 - 2028 (USD Million)
Fig. 51 Global fill finish market estimates and forecast 2017 - 2028 (USD Million)
Fig. 52 Viral vector production (research use) market: Application outlook and key takeaways
Fig. 53 Viral vector production (research use) market: Application movement analysis
Fig. 54 Percentage of viral vector in gene therapy clinical trials as of April 2019
Fig. 55 Viral vector production (research use) market estimates and forecast for gene and cell therapy development, 2017 - 2028 (USD Million)
Fig. 56 Viral vector manufacturing market(research use) estimates and forecast for vaccine development, 2017 - 2028 (USD Million)
Fig. 57 Viral vector production (research use) market estimates and forecast for biopharmaceutical and pharmaceutical discovery, 2017 - 2028 (USD Million)
Fig. 58 Viral vector production (research use) market estimates and forecast for biomedical research, 2017 - 2028 (USD Million)
Fig. 59 Viral vector production (research use) market: End-use outlook and key takeaways
Fig. 60 Viral vector production (research use) market: End-use movement analysis
Fig. 61 Global viral vector production (research use) market estimates and forecast for pharmaceutical and biopharmaceutical companies 2017 - 2028 (USD Million)
Fig. 62 Global viral vector production (research use) market estimates and forecast, for research institutes 2017 - 2028
Fig. 63 Viral vector production (research use) market: Regional outlook and key takeaways
Fig. 64 Viral vector production (research use) market: Regional movement analysis
Fig. 65 North America viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 66 U.S. viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 67 Canada. viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 68 Europe viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 69 Germany viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 70 U.K. viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 71 France viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 72 Spain viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 73 Italy viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 74 Asia Pacific viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 75 China viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 76 Japan viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 77 India viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 78 South Korea viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 79 Australia viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 80 Latin America viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 81 Brazil viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 82 Mexico viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 83 MEA viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 84 South Africa viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 85 Saudi Arabia viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 86 New developments-based initiatives by key market participants
Fig. 87 Impact analysis of new developments by key market participants
Fig. 88 Company/competition categorization
Fig. 89 Distribution by location of manufacturing facility
Fig. 90 CDMOs operating in China
Fig. 91 Distribution by the scale of production
Fig. 92 Company mapping analysis, by manufacturing facility, type of organization, and purpose of production
Fig. 93 List of companies with a portfolio comprising antibody & viral vector-based therapeutic candidates
Fig. 94 Key public companies: Heat map analysis
Fig. 95 List of companies with a portfolio comprising antibody & viral vector-based therapeutic candidates
Fig. 96 Synergy analysis: Major deals & strategic alliances
Fig. 97 Participant categorization
Fig. 98 Strategy framework
Fig. 99 SWOT analysis Merck
Fig. 100 SWOT analysis Lonza
Fig. 101 SWOT analysis FUJIFILM
Fig. 102 SWOT analysis Cobra Biologics
Fig. 103 SWOT analysis Brammer Bio
Fig. 104 SWOT analysis Waisman Biomanufacturing
Fig. 105 SWOT analysis Genezen
Fig. 106 SWOT analysis YPOSKESI
Fig. 107 SWOT analysis ABL Inc.
Fig. 108 SWOT analysis Novasep Holding S.A.S
Fig. 109 SWOT analysis Orgenesis Biotech Israel Ltd
Fig. 110 SWOT analysis Vigene Biosciences
Fig. 111 SWOT analysis GE Healthcare
Fig. 112 SWOT analysis Cevec Pharmaceuticals GmbH
Fig. 113 SWOT analysis Batavia Biosciences
Fig. 114 SWOT analysis Biovion
Fig. 115 SWOT analysis Wuxi AppTec Co Ltd.
Fig. 116 SWOT analysis VGXI Inc.
Fig. 117 SWOT analysis Paragon Servicest Inc.
Fig. 118 SWOT analysis Lentigen Technology
Fig. 119 SWOT analysis Sirion Biotech GmbH
Fig. 120 SWOT analysis Virovek Incorporation
Fig. 121 SWOT analysis BioNTech
Fig. 122 SWOT analysis VIVEbiotech
Fig. 123 SWOT analysis Creative Biogene
Fig. 124 SWOT analysis Vibalogics GmbH
Fig. 125 SWOT analysis Takara BioInc.
Fig. 126 SWOT analysis cell and gene therapy catapult
Fig. 127 SWOT analysis Bluebird Bio Inc.
Fig. 128 SWOT analysis Addgene Inc.
Fig. 129 SWOT analysis Aldevron LLC
Fig. 130 SWOT analysis Audentis
Fig. 131 SWOT analysis BioMarin Pharmaceuticals
Fig. 132 SWOT analysis REGENXBIO Inc.
Fig. 2 Market research process
Fig. 3 Information procurement
Fig. 4 Primary research process
Fig. 5 Primary research validation
Fig. 6 Market research approaches
Fig. 7 Value-chain-based sizing & forecasting
Fig. 8 QFD modeling
Fig. 9 Market formulation and validation
Fig. 10 Vector type characteristic analysis
Fig. 11 QFD model assessment for vector type
Fig. 12 Vector type characteristic analysis
Fig. 13 Lentiviral vectors versus AAV vectors
Fig. 14 Global number of AAV- and lentivirus-based clinical trials
Fig. 15 Gene therapy approvals versus clinical trials
Fig. 16 Some major industry sponsors of CAR T cell clinical trials, as of September 2019
Fig. 17 Distribution of the sponsors of advanced therapies clinical trials, 2016
Fig. 18 Distribution of noncommercial sponsors of ATMP clinical trials, 2016
Fig. 19 Gene therapy approvals versus clinical trials
Fig. 20 Penetration by end use settings
Fig. 21 Regional analysis for rare disease genetic testing market
Fig. 22 U.K. cell and gene therapy manufacturing capacity, 2016 to 2019
Fig. 23 Number of gene therapy sites across gene therapy and multifunctional facilities in the U.K., 2019
Fig. 24 Market trends & outlook
Fig. 25 Market segmentation & scope
Fig. 26 Market driver relevance analysis (Current & future impact)
Fig. 27 Gene therapy clinical trials
Fig. 28 Viral vectors used in clinical trials
Fig. 29 Key strategies by market players to drive success in the gene therapy arena
Fig. 30 Recent and upcoming approved gene transfer-mediated programs
Fig. 31 Market restraint relevance analysis (Current & future impact)
Fig. 32 Various ethical questions raised by gene therapy
Fig. 33 Major challenges in large-scale vector manufacturing
Fig. 34 Penetration & growth prospect mapping for vector type, 2020
Fig. 35 Porter’s Five Forces Analysis
Fig. 36 SWOT analysis, by factor (Political & Legal, Economic and Technological)
Fig. 37 Viral vector production (research use) market: Vector type outlook and key takeaways
Fig. 38 Viral vector production (research use) market: Vector type movement analysis
Fig. 39 Viral vector production (research use) market estimates and forecast for Adenovirus 2017 - 2028 (USD Million)
Fig. 40 Viral vector production (research use) market estimates and forecast for a retrovirus, 2017 - 2028 (USD Million)
Fig. 41 Viral vector production (research use) market estimates and forecast for AAV, 2017 - 2028 (USD Million)
Fig. 42 Viral vector production (research use) market estimates and forecast for Lentivirus, 2017 - 2028 (USD Million)
Fig. 43 Viral vector production (research use) market estimates and forecast for Others, 2017 - 2028 (USD Million)
Fig. 44 Viral vector production (research use) market: Workflow outlook and key takeaways
Fig. 45 Viral vector production (research use) market: Workflow movement analysis
Fig. 46 Global upstream processing market estimates and forecast 2017 - 2028 (USD Million)
Fig. 47 Global vector amplification and expansion market estimates and forecast 2017 - 2028 (USD Million)
Fig. 48 Global vector recovery/harvesting market estimates and forecast 2017 - 2028 (USD Million)
Fig. 49 Global downstream processing market estimates and forecast, for 2017 - 2028 (USD Million)
Fig. 50 Global purification market estimates and forecast 2017 - 2028 (USD Million)
Fig. 51 Global fill finish market estimates and forecast 2017 - 2028 (USD Million)
Fig. 52 Viral vector production (research use) market: Application outlook and key takeaways
Fig. 53 Viral vector production (research use) market: Application movement analysis
Fig. 54 Percentage of viral vector in gene therapy clinical trials as of April 2019
Fig. 55 Viral vector production (research use) market estimates and forecast for gene and cell therapy development, 2017 - 2028 (USD Million)
Fig. 56 Viral vector manufacturing market(research use) estimates and forecast for vaccine development, 2017 - 2028 (USD Million)
Fig. 57 Viral vector production (research use) market estimates and forecast for biopharmaceutical and pharmaceutical discovery, 2017 - 2028 (USD Million)
Fig. 58 Viral vector production (research use) market estimates and forecast for biomedical research, 2017 - 2028 (USD Million)
Fig. 59 Viral vector production (research use) market: End-use outlook and key takeaways
Fig. 60 Viral vector production (research use) market: End-use movement analysis
Fig. 61 Global viral vector production (research use) market estimates and forecast for pharmaceutical and biopharmaceutical companies 2017 - 2028 (USD Million)
Fig. 62 Global viral vector production (research use) market estimates and forecast, for research institutes 2017 - 2028
Fig. 63 Viral vector production (research use) market: Regional outlook and key takeaways
Fig. 64 Viral vector production (research use) market: Regional movement analysis
Fig. 65 North America viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 66 U.S. viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 67 Canada. viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 68 Europe viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 69 Germany viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 70 U.K. viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 71 France viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 72 Spain viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 73 Italy viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 74 Asia Pacific viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 75 China viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 76 Japan viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 77 India viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 78 South Korea viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 79 Australia viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 80 Latin America viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 81 Brazil viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 82 Mexico viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 83 MEA viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 84 South Africa viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 85 Saudi Arabia viral vector production (research use) market estimates and forecast, 2017 - 2028 (USD Million)
Fig. 86 New developments-based initiatives by key market participants
Fig. 87 Impact analysis of new developments by key market participants
Fig. 88 Company/competition categorization
Fig. 89 Distribution by location of manufacturing facility
Fig. 90 CDMOs operating in China
Fig. 91 Distribution by the scale of production
Fig. 92 Company mapping analysis, by manufacturing facility, type of organization, and purpose of production
Fig. 93 List of companies with a portfolio comprising antibody & viral vector-based therapeutic candidates
Fig. 94 Key public companies: Heat map analysis
Fig. 95 List of companies with a portfolio comprising antibody & viral vector-based therapeutic candidates
Fig. 96 Synergy analysis: Major deals & strategic alliances
Fig. 97 Participant categorization
Fig. 98 Strategy framework
Fig. 99 SWOT analysis Merck
Fig. 100 SWOT analysis Lonza
Fig. 101 SWOT analysis FUJIFILM
Fig. 102 SWOT analysis Cobra Biologics
Fig. 103 SWOT analysis Brammer Bio
Fig. 104 SWOT analysis Waisman Biomanufacturing
Fig. 105 SWOT analysis Genezen
Fig. 106 SWOT analysis YPOSKESI
Fig. 107 SWOT analysis ABL Inc.
Fig. 108 SWOT analysis Novasep Holding S.A.S
Fig. 109 SWOT analysis Orgenesis Biotech Israel Ltd
Fig. 110 SWOT analysis Vigene Biosciences
Fig. 111 SWOT analysis GE Healthcare
Fig. 112 SWOT analysis Cevec Pharmaceuticals GmbH
Fig. 113 SWOT analysis Batavia Biosciences
Fig. 114 SWOT analysis Biovion
Fig. 115 SWOT analysis Wuxi AppTec Co Ltd.
Fig. 116 SWOT analysis VGXI Inc.
Fig. 117 SWOT analysis Paragon Servicest Inc.
Fig. 118 SWOT analysis Lentigen Technology
Fig. 119 SWOT analysis Sirion Biotech GmbH
Fig. 120 SWOT analysis Virovek Incorporation
Fig. 121 SWOT analysis BioNTech
Fig. 122 SWOT analysis VIVEbiotech
Fig. 123 SWOT analysis Creative Biogene
Fig. 124 SWOT analysis Vibalogics GmbH
Fig. 125 SWOT analysis Takara BioInc.
Fig. 126 SWOT analysis cell and gene therapy catapult
Fig. 127 SWOT analysis Bluebird Bio Inc.
Fig. 128 SWOT analysis Addgene Inc.
Fig. 129 SWOT analysis Aldevron LLC
Fig. 130 SWOT analysis Audentis
Fig. 131 SWOT analysis BioMarin Pharmaceuticals
Fig. 132 SWOT analysis REGENXBIO Inc.
Companies Mentioned
- Merck kgaa
- Lonza
- FUJIFILM Diosynth Biotechnologies U.S.A., Inc.
- Cobra Biologics Ltd.
- Thermofisher Scientific Inc.
- Waisman Biomanufacturing
- genezen laboratories
- YPOSKESI
- Advanced BioScience Laboratories, Inc. (ABL inc.)
- Novasep Holding s.a.s.
- Orgenesis Biotech Israel Ltd (formerly ATVIO Biotech ltd.)
- Vigene biosciences Inc.
- General electric company (ge healthcare).
- CEVEC. pharmaceuticals gmbh
- Batavia Biosciences B.v.
- Biovion oy
- Wuxi AppTec Co., Ltd.
- VGXI, Inc.
- Catalent Inc.
- Miltenyi Biotec gmbh
- sirion biotech gmbh.
- virovek incorporation
- BioNTech IMFS GmbH
- VIVEbiotech s.l.
- creative biogene
- Vibalogics GmbH
- Takara Bio Inc.
- Cell and Gene Therapy Catapult
- Bluebird Bio Inc.
- Addgene Inc.
- Aldevron LLc.
- Astellas Pharma, Inc.
- BioMarin Pharmaceutical, Inc.
- RegenxBio, Inc.
Methodology

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | May 2021 |
| Forecast Period | 2021 - 2028 |
| Estimated Market Value ( USD | $ 1.3 billion |
| Forecasted Market Value ( USD | $ 3.9 billion |
| Compound Annual Growth Rate | 17.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 34 |