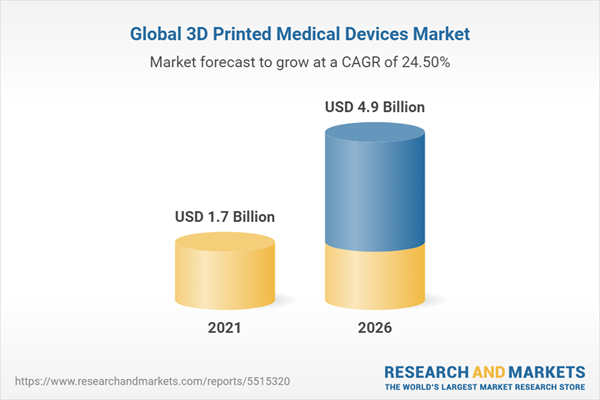
The global market for 3D printed medical devices - should grow from $1.7 billion in 2021 to $4.9 billion by 2026, at a compound annual growth rate (CAGR) of 24.5% for the period of 2021-2026.
The North American market for 3D printed medical devices should grow from $632.6 million in 2021 to $1.9 billion by 2026, at a CAGR of 24.9% for the period of 2021-2026.
The Asia-Pacific market for 3D printed medical devices should grow from $367.5 million in 2021 to $1.2 billion by 2026, at a CAGR of 26.7% for the period of 2021-2026.
Report Scope
The taxonomy of the market by component, material, technology, application, end-user and the geographical region was considered for this study. Using 2020 as the base year, the report provides estimated market data for the forecast period of 2021 through 2026). Market values have been estimated based on total revenue from hardware, software and services, and material provider revenues.
This report also focuses on the drivers and challenges impacting the market. The report explores current trends in the 3D printed medical devices market, concluding with an analysis of the vendor landscape and profiles of the major players in the global market.
The Report Includes
- 55 data tables and 20 additional tables
- An up-to-date review of the global market for 3D printed medical devices
- Analyses of the global market trends, with data from 2020, estimates for 2021, 2022 and 2024, along with projections of compound annual growth rates (CAGRs) through 2026
- Highlights of the market potential for 3D printing technologies in orthopedic and cranial implants, surgical instruments and dental restorations; opportunities and gaps estimating current and future demand; and impact of COVID-19 on the progress of this market
- Evaluation and forecast the global 3D printed medical devices market size in dollar value terms, and corresponding market share analysis by technology, material, application, component, end-user and geographic region
- Discussion of key market dynamics (DROs), technology advancements, regulatory scenario, pricing analysis in the global market and its sub-segments
- Identification of the major stakeholders and analysis of their competitive landscape based on recent developments and segmental revenues
- Insight into the recent R&D activities, mergers and acquisitions, collaboration agreements, and competitive environment of the leading vendor companies
- Company profile descriptions of the leading market players including 3D Systems, EnvisionTec, EOS, General Electric Additive, Materialise N.V.,and Stratasys Ltd.
Table of Contents
Chapter 1 Introduction
- Market Definition
- Study Goals and Objectives
- Reasons for Doing This Study
- What's New in This Update
- Scope of Report
- Intended Audiences
- Information Sources
- Methodology
- Geographic Breakdown
- Analyst's Credentials
- Custom Research
- Related Reports
Chapter 2 Summary and Highlights
Chapter 3 Market and Technology Background
- Value Chain Analysis
- History of 3D Printing
- Current Market Trends
- 3D Printing Methods
- Market Regulations
- Patent Analysis
- Market Drivers
- Technological Developments
- Government Initiatives
- Market Challenges
- Regulatory Challenges
- Need for Skilled Workforce
- High Initial Setup Costs
- Market Opportunities
- Direct Digital Manufacturing
- Emergence of Desktop Printers
- 4D Printing
- Porter's Five Forces Analysis
- Power of Suppliers
- Power of Buyers
- Threat of New Entrants
- Threat of Substitutes
- Degree of Competition
- Impact of COVID-19
- Future of 3D Printed Medical Devices
- Needs of 3D Printing
- Patient-specific Devices
- Generic Devices
- Cloud Manufacturing
Chapter 4 Market Breakdown by Component
- Software and Services
- Materials
- Equipment/Hardware
Chapter 5 Market Breakdown by Technology
- Introduction
- Material Extrusion
- Powder Bed Fusion
- Vat Polymerization
- Material Jetting
- Binder Jetting
- Directed Energy Deposition
- Sheet Lamination
- Other Technologies
Chapter 6 Market Breakdown by Material
- Metals
- Polymers
- Ceramics
- Biomaterials
- Other Materials
Chapter 7 Market Breakdown by Application
- Prototyping, Design and Development
- Anatomical Models
- Surgical Instruments
- Prosthetics
- Implants
- Dental
- Bioprinting
- Other Applications
Chapter 8 Market Breakdown by End User
- Introduction
- Hospitals and Clinics
- Academic and Research Institutions
- Pharmaceutical and Medical Device Manufacturers
- Other End Users
Chapter 9 Market Breakdown by Region
- North America
- Europe
- Asia-Pacific
- RoW
Chapter 10 Competitive Landscape
- Market Share Analysis
Chapter 11 Company Profiles
- Key Market Players
- 3D Systems Corp.
- Envisiontec Inc.
- EOS Gmbh
- General Electric Additive
- Materialise N.V.
- Renishaw Plc
- Stratasys Ltd.
- Other Players
- 3D Bioprinting Solutions
- 3D Lifeprints
- 3D Orthotics Ltd.
- Additive Orthopaedics Llc
- Adeiss
- Align Technology
- Amedica Corp.
- Anatomicsrx
- Anatomikmodeling
- Anika Therapeutics Inc.
- Antleron
- Aspect Biosystems
- Bioarchitects
- Biolase Inc.
- Breca Health Care
- Carbon Inc.
- Conformis
- Core3Dcentres
- Cyfuse Biomedical
- Dentis Usa
- Dentsply Sirona
- Depuy Synthes
- Dunlee
- Esstech Inc.
- Evo Dental
- Exone
- Fast Radius
- Formlabs Inc.
- Handsmith Inc.
- Hewlett Packard Inc.
- Invent Medical
- Koln 3D Technology (Industry) Co. Ltd.
- Lima Corp.
- Limbforge
- Limbitless Solutions
- Luminex Corp.
- Maxon Computer Gmbh
- Medicrea
- Medprin Biotech Gmbh
- Medtronic
- Microfabrica Inc.
- MT Ortho Srl
- Norman Noble Am
- Novax Dma
- Onkos Surgical Inc.
- Open Bionics
- Optomec Inc.
- Organovo Inc.
- Osseus Fusion Systems
- Oxford Performance Materials
- Resound U.S.
- Rokit Inc.
- Royal Dsm (Koninklijke Dsm N.V.)
- Salvatore Dental
- Si-Bone Inc.
- Sinterex
- Smile Direct Club
- Smith & Nephew Plc
- Solidscape Inc.
- Sonova Ag
- Standard Cyborg Inc.
- Stryker Corp.
- Tangible Solutions
- Unyq Design Inc.
- Xometry
- Zimmer Biomet
Chapter 12 Appendix: Abbreviations and Terms
List of Tables
Summary Table: Global Market for 3D Printed Medical Devices, by Region, Through 2026
Table 1: 3D Printed Medical Devices History
Table 2: Advantages of 3D Printing over Traditional Methods
Table 3: 3D Printing Methods and Materials
Table 4: 3D Printed Medical Devices Regulations, by Region
Table 5: Porter's Five Forces Analysis: Overview
Table 6: Global Market for 3D Printed Medical Devices, by Component, Through 2026
Table 7: Global Market for Software and Services for 3D Printed Medical Devices, by Region, Through 2026
Table 8: Global Market for Materials for 3D Printed Medical Devices, by Region, Through 2026
Table 9: Global Market for Equipment/Hardware for 3D Printed Medical Devices, by Region, Through 2026
Table 10: Snapshot of 3D Printing Technologies
Table 11: Global Market for 3D Printed Medical Devices, by Technology, Through 2026
Table 12: Global Market for Material Extrusion for 3D Printed Medical Devices, by Region, Through 2026
Table 13: Global Market for Powder Bed Fusion for 3D Printed Medical Devices, by Region, Through 2026
Table 14: Global Market for Vat Polymerization for 3D Printed Medical Devices, by Region, Through 2026
Table 15: Global Market for Material Jetting for 3D Printed Medical Devices, by Region, Through 2026
Table 16: Global Market for Binder Jetting for 3D Printed Medical Devices, by Region, Through 2026
Table 17: Global Market for Direct Energy Deposition for 3D Printed Medical Devices, by Region, Through 2026
Table 18: Global Market for Sheet Lamination for 3D Printed Medical Devices, by Region, Through 2026
Table 19: Global Market for Other Technologies for 3D Printed Medical Devices, by Region, Through 2026
Table 20: Global Market for 3D Printed Medical Devices, by Material, Through 2026
Table 21: Global Market for Metals in 3D Printed Medical Devices, by Region, Through 2026
Table 22: Global Market for Polymers in 3D Printed Medical Devices, by Region, Through 2026
Table 23: Global Market for Ceramics in 3D Printed Medical Devices, by Region, Through 2026
Table 24: Market for Biomaterials in 3D Printed Medical Devices, by Region, Through 2026
Table 25: Global Market for Other Materials in 3D Printed Medical Devices, by Region, Through 2026
Table 26: Global Market for 3D Printed Medical Devices, by Application, Through 2026
Table 27: Global Market for Prototyping, Design and Development of 3D Printed Medical Devices, by Material, Through 2026
Table 28: Global Market for Prototyping, Design and Development of 3D Printed Medical Devices, by Technology, Through 2026
Table 29: Global Market for Prototyping, Design and Development of 3D Printed Medical Devices, by Region, Through 2026
Table 30: Global Market for 3D Printed Anatomical Models, by Material, Through 2026
Table 31: Global Market for 3D Printed Anatomical Models, by Technology, Through 2026
Table 32: Global Market for 3D Printed Anatomical Models, by Region, Through 2026
Table 33: Global Market for 3D Printed Surgical Instruments, by Material, Through 2026
Table 34: Global Market for 3D Printed Surgical Instruments, by Technology, Through 2026
Table 35: Global Market for 3D Printed Surgical Instruments, by Region, Through 2026
Table 36: Global Market for 3D Printed Prosthetics, by Material, Through 2026
Table 37: Global Market for 3D Printed Prosthetics, by Technology, Through 2026
Table 38: Global Market for 3D Printed Prosthetics, by Region, Through 2026
Table 39: Global Market for 3D Printed Implants, by Material, Through 2026
Table 40: Global Market for 3D Printed Implants, by Technology, Through 2026
Table 41: Global Market for 3D Printed Implants, by Region, Through 2026
Table 42: Global Market for 3D Printed Dental Products, by Material, Through 2026
Table 43: Global Market for 3D Printed Dental Products, by Technology, Through 2026
Table 44: Global Market for 3D Printed Dental Products, by Region, Through 2026
Table 45: Global Market for 3D Bioprinting, by Material, Through 2026
Table 46: Global Market for 3D Bioprinting, by Technology, Through 2026
Table 47: Global Market for 3D Bioprinting, by Region, Through 2026
Table 48: Global Market for Other Applications for 3D Printed Medical Devices, by Material, Through 2026
Table 49: Global Market for Other Applications for 3D Printed Medical Devices, by Technology, Through 2026
Table 50: Global Market for Other Applications for 3D Printed Medical Devices, by Region, Through 2026
Table 51: Global Market for 3D Printed Medical Devices, by End User, Through 2026
Table 52: Global Market for 3D Printed Medical Devices Used in Hospitals and Clinics, by Region, Through 2026
Table 53: Global Market for 3D Printed Medical Devices Used in Academic and Research Institutions, by Region, Through 2026
Table 54: Global Market for 3D Printed Medical Devices Used by Pharmaceutical and Medical Device Manufacturers, by Region, Through 2026
Table 55: Global Market for 3D Printed Medical Devices Used by Other End Users, by Region, Through 2026
Table 56: Regulatory Scenarios, by Region
Table 57: Global Market for 3D Printed Medical Devices, by Region, Through 2026
Table 58: North American Market for 3D Printed Medical Devices, by Country, Through 2026
Table 59: European Market for 3D Printed Medical Devices, by Country, Through 2026
Table 60: Asia-Pacific Market for 3D Printed Medical Devices, by Country, Through 2026
Table 61: RoW Market for 3D Printed Medical Devices, by Region, Through 2026
Table 62: 3D Systems: Product Portfolio
Table 63: 3D Systems: Recent Developments
Table 64: EnvisionTec GmbH: Product Portfolio
Table 65: EnvisionTec GmbH: Recent Developments
Table 66: EOS GmbH: Product Portfolio
Table 67: GE Additive: Product Portfolio
Table 68: Materialise N.V.: Product Portfolio
Table 69: Materialise N.V.: Recent Developments
Table 70: Renishaw PLC: Product Portfolio
Table 71: Stratasys: Product Portfolio
Table 72: Stratasys: Recent Developments
Table 73: Abbreviations Used in this Report
Table 74: Glossary of Terms
Table 75: Sources Used in this Report
List of Figures
Summary Figure: Global Market for 3D Printing of Medical Devices, 2020-2026
Figure 1: Supply Chain for 3D Printed Medical Devices
Figure 2: Value Chain for 3D Printed Medical Devices
Figure 3: 3D Printing Process
Figure 4: Patents for 3D Printed Medical Devices, 2016-2021
Figure 5: Porter's Five Forces Analysis, 3D Printed Medical Device Market
Figure 6: Power of Suppliers
Figure 7: Power of Buyers
Figure 8: Threat of New Entrants
Figure 9: Threat of Substitutes
Figure 10: Degree of Competition
Figure 11: Global Market for 3D Printed Medical Devices, by Component, 2020
Figure 12: 3D Printed Medical Devices Software Stakeholders
Figure 13: 3D Printed Medical Devices Materials Stakeholders
Figure 14: 3D Printed Medical Devices Hardware Stakeholders
Figure 15: Ranking of 3D Printing Methods, by Pricing
Figure 16: Global Market for 3D Printed Medical Devices, by Technology, 2026
Figure 17: Global Market for 3D Printed Medical Devices, by Material, 2021
Figure 18: Global Market for 3D Printed Medical Devices, by Application, 2021
Figure 19: Global Market for 3D Printed Medical Devices, by End User, 2020
Figure 20: Global Market for 3D Printed Medical Devices, by Region, 2020
Figure 21: Global Market for 3D Printed Medical Devices, 2020
Figure 22: Strategies Used by Leading Companies in the Market for 3D Printed Medical Devices, 2018-2020
Figure 23: 3D Systems: Financials, 2018-2020
Figure 24: 3D Systems: Revenue Share, by Business Segment, 2020
Figure 25: General Electric: Financials, 2018-2020
Figure 26: General Electric: Revenue Share, by Business Segment, 2020
Figure 27: General Electric: Revenue Share, by Region, 2020
Figure 28: Materialise N.V.: Financials, 2018-2020
Figure 29: Materialise N.V.: Revenue Share, by Business Segment, 2020
Figure 30: Materialise N.V.: Revenue Share, by Region, 2020
Figure 31: Renishaw PLC: Financials, 2018-2020
Figure 32: Renishaw PLC: Revenue Share, by Region, 2020
Figure 33: Stratasys: Financials, 2018-2020
Figure 34: Stratasys: Revenue Share, by Business Segment, 2020
Figure 35: Stratasys: Revenue Share, by Region, 2020
Executive Summary
3D printed medical devices are a segment of the larger medical device industry. These devices can be manufactured at end-user facilities with 3D printers and compatible scanners and software or purchased through a 3D printing vendor, which uses transmitted patient scans digitally to print the device that is then delivered to the healthcare provider. There are numerous end-user markets for 3D printed medical devices. Hospitals, physical therapist clinics, outpatient care centers and physicians’ offices worldwide use millions of medical devices for implanting joints, surgeries (instruments), joint braces (orthopedics) and prosthetics. 3D printed orthodontic appliances are becoming increasingly common and are manufactured in-house at a growing rate.
The term 3D printing originally referred to a process that deposits a binder material onto a powder bed with inkjet printer heads layer by layer. More recently, the term is being used to encompass a broader variety of additive manufacturing techniques. While the industry is enjoying dramatic success, there are hurdles to maintaining this in the future. Insiders recognize the continued need for global industry standards, both in terminology and production methodologies but also in testing standards and safety standards. Many of these standards are being applied to the industry by government agencies that test devices and give approval to new devices, but it is the industry companies that will work together to enhance these standards.
Government regulations are another hurdle for industry companies. The U.S. Food and Drug Administration (FDA) approves devices and sets guidelines that companies must adhere to when submitting new devices to testing. The FDA issued guidance for 3D printing medical device manufacturers in 2017 which serves as technical considerations for additive manufactured devices. The guidance is intended for public health safety and product development.
Companies Mentioned
- 3D Bioprinting Solutions
- 3D Lifeprints
- 3D Orthotics Ltd.
- 3D Systems Corp.
- Additive Orthopaedics LLC
- Adeiss
- Align Technology
- Amedica Corp.
- Anatomicsrx
- Anatomikmodeling
- Anika Therapeutics Inc.
- Antleron
- Aspect Biosystems
- Bioarchitects
- Biolase Inc.
- Breca Health Care
- Carbon Inc.
- Conformis
- Core3Dcentres
- Cyfuse Biomedical
- Dentis Usa
- Dentsply Sirona
- Depuy Synthes
- Dunlee
- Envisiontec Inc.
- EOS Gmbh
- Esstech Inc.
- Evo Dental
- Exone
- Fast Radius
- Formlabs Inc.
- General Electric Additive
- Handsmith Inc.
- Hewlett Packard Inc.
- Invent Medical
- Koln 3D Technology (Industry) Co. Ltd.
- Lima Corp.
- Limbforge
- Limbitless Solutions
- Luminex Corp.
- Materialise N.V.
- Maxon Computer Gmbh
- Medicrea
- Medprin Biotech Gmbh
- Medtronic
- Microfabrica Inc.
- MT Ortho Srl
- Norman Noble Am
- Novax Dma
- Onkos Surgical Inc.
- Open Bionics
- Optomec Inc.
- Organovo Inc.
- Osseus Fusion Systems
- Oxford Performance Materials
- Renishaw Plc
- Resound U.S.
- Rokit Inc.
- Royal Dsm (Koninklijke Dsm N.V.)
- Salvatore Dental
- Si-Bone Inc.
- Sinterex
- Smile Direct Club
- Smith & Nephew Plc
- Solidscape Inc.
- Sonova Ag
- Standard Cyborg Inc.
- Stratasys Ltd.
- Stryker Corp.
- Tangible Solutions
- Unyq Design Inc.
- Xometry
- Zimmer Biomet
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 115 |
| Published | December 2021 |
| Forecast Period | 2021 - 2026 |
| Estimated Market Value ( USD | $ 1.7 Billion |
| Forecasted Market Value ( USD | $ 4.9 Billion |
| Compound Annual Growth Rate | 24.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 73 |