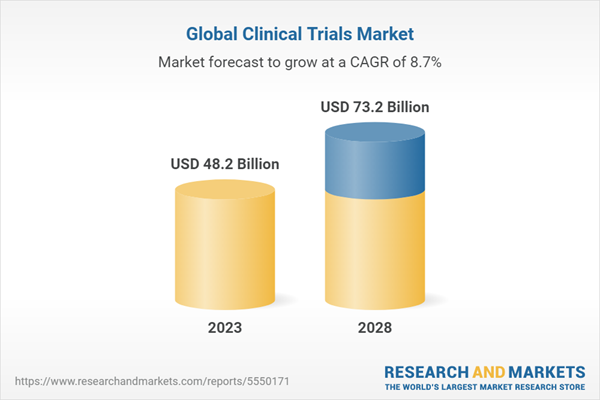
1 Introduction
1.1 Study Objectives
1.2 Market Definition
1.2.1 Inclusions and Exclusions
1.3 Market Scope
1.3.1 Markets Covered
1.3.2 Years Considered
1.3.3 Currency Considered
1.4 Limitations
1.5 Stakeholders
1.6 Summary of Changes
1.6.1 Recession Impact
2 Research Methodology
2.1 Research Data
Figure 1 Research Design
2.1.1 Secondary Data
2.1.2 Primary Data
Figure 2 Clinical Trials Market: Breakdown of Primaries
2.2 Market Size Estimation
Figure 3 Market Size Estimation: Supply-Side Analysis, 2022
Figure 4 Market Size Estimation: Approach 1 (Revenue Share Analysis), 2022
Figure 5 Illustrative Example of Iqvia: Revenue Share Analysis, 2022
2.2.1 Insights from Primaries
Figure 6 Market Validation from Primary Experts
2.2.2 Segment Assessment (By Phase, Service Type, Therapeutic Area, and Application)
Figure 7 Market Size Estimation Methodology: Top-Down Approach
2.2.3 Segment Assessment (By Phase, Service Type, and Therapeutic Area)
2.3 Growth Forecast
Figure 8 Market: CAGR Projections, 2023-2028
Figure 9 Clinical Trials Industry: Growth Analysis of Drivers, Opportunities, and Challenges
2.4 Market Breakdown and Data Triangulation
Figure 10 Data Triangulation Methodology
2.5 Research Assumptions
2.6 Risk Analysis
2.7 Market: Recession Impact Analysis
Table 1 Global Inflation Rate Projections, 2021-2028 (% Growth)
Table 2 US Health Expenditure, 2019-2022 (USD Million)
Table 3 US Health Expenditure, 2023-2030 (USD Million)
3 Executive Summary
Figure 11 Clinical Trials Market, by Phase, 2023 Vs. 2028 (USD Million)
Figure 12 Market, by Therapeutic Area, 2023 Vs. 2028 (USD Million)
Figure 13 Market, by Application, 2023 Vs. 2028 (USD Million)
Figure 14 Market, by Service Type, 2023 Vs. 2028 (USD Million)
Figure 15 Geographical Snapshot: Clinical Trials Industry
4 Premium Insights
4.1 Clinical Trials Market Overview
Figure 16 Rising Investments in Pharmaceutical Industry to Drive Market
4.2 North America: Market Share, by Phase & Country (2022)
Figure 17 Phase Iii Segment Accounted for Largest Share of North American Market in 2022
4.3 Bioanalytical Services Market, by Type, 2023 Vs. 2028 (USD Billion)
Figure 18 Cell-Based Assays to Dominate Market Till 2028
4.4 Market Share, by Therapeutic Area, 2023-2028
Figure 19 Oncology to Hold Largest Market Share Till 2028
4.5 Clinical Trials Industry: Geographic Growth Opportunities
Figure 20 Asia-Pacific to Register Highest Growth from 2023 to 2028
5 Market Overview
5.1 Introduction
5.2 Market Dynamics
Figure 21 Clinical Trials Market: Drivers, Opportunities, and Challenges
Table 4 Market: Impact Analysis of Drivers, Restraints, Opportunities, and Challenges
5.2.1 Drivers
5.2.1.1 Increasing Drugs in Pipeline and Rising Investments in Pharmaceutical R&D
Figure 22 Increase in Global Pharmaceutical R&D Spending, 2014-2028
Figure 23 Active Pharmaceutical Pipeline, 2011-2023
5.2.1.2 Increasing Number of Clinical Trials
Figure 24 Number of Clinical Trials Registered Worldwide (2000-2022)
5.2.1.3 High Cost of In-House Drug Development
Figure 25 Distribution of In-House and Outsourcing Services Expenditure in Global Pharma Market, 2014-2023 (% Distribution)
5.2.1.4 Rising Prevalence of Orphan and Rare Diseases
Figure 26 Overview of Top 20 Companies Focusing on Rare Diseases (As of 2023)
Figure 27 Percentage of New Active Substances Launches with Orphan Drug Designation, 2012-2022
5.2.2 Opportunities
5.2.2.1 Favorable Outlook for Biologics and Biosimilars
Figure 28 Total Number of New Drugs (New Chemical Entities and Biologics) Approved by Us Fda (2015-2022)
Table 5 List of Biologics Approved by Us Fda, 2022
Figure 29 Biosimilar Approvals and Launches, 2015-2022
5.2.2.2 Rising Demand for Specialized Testing Services
5.2.2.3 Need for Novel Clinical Trial Designs for Complex Cell and Gene Therapies
Figure 30 Number of Ongoing Clinical Trials, by Phase and Therapeutic Approach (As of April 2023)
Figure 31 Gene and Cell Therapy Developers, Clinical Trials, and Investments, by Region (As of 2022)
5.2.3 Challenges
5.2.3.1 Shortage of Skilled Professionals for Clinical Trials
5.2.3.2 Need for Unique Testing Approaches for Innovative Molecules
5.2.4 Trends
5.2.4.1 Adoption of Ai-Based Tools for Drug Discovery
5.2.4.2 Increasing Outsourcing Activities in Emerging Asian Economies
5.2.4.3 Integrated End-To-End R&D Service Solutions
5.3 Trends/Disruptions Impacting Customer's Business
Figure 32 Revenue Shift & New Pockets for Clinical Trial Service Providers
5.4 Value Chain Analysis
Figure 33 Value Chain Analysis of Clinical Trials
5.5 Indicative Pricing Analysis
Table 6 Phase 3 Oncology Clinical Trial Cost (Us and European Countries)
Table 7 Phase I Oncology Clinical Trial Cost (Spain-Europe)
5.6 Ecosystem Analysis
Figure 34 Ecosystem Analysis: Market
Table 8 Overall Market Ecosystem for Clinical Trials
5.7 Technology Analysis
Figure 35 Importance of Technology Adoption in Clinical Trials
5.8 Porter's Five Forces Analysis
Table 9 Market: Porter's Five Forces Analysis
5.8.1 Threat of New Entrants
5.8.2 Threat of Substitutes
5.8.3 Bargaining Power of Buyers
5.8.4 Bargaining Power of Suppliers
5.8.5 Degree of Competition
5.9 Regulatory Analysis
5.9.1 Regulatory Bodies, Government Agencies, and Other Organizations
Table 10 North America: Regulatory Bodies, Government Agencies, and Other Organizations
Table 11 Europe: Regulatory Bodies, Government Agencies, and Other Organizations
Table 12 Asia-Pacific: Regulatory Bodies, Government Agencies, and Other Organizations
Table 13 RoW: Regulatory Bodies, Government Agencies, and Other Organizations
Table 14 Market Regulatory Scenario, by Country/Region
5.10 Key Conferences and Events (2023-2024)
Table 15 Clinical Trials Industry: Detailed List of Conferences & Events (2023-2024)
5.11 Key Stakeholders & Buying Criteria
5.11.1 Key Stakeholders in Buying Process
Figure 36 Influence of Stakeholders on Buying Process of Clinical Trials
5.11.2 Buying Criteria for Clinical Trials
Figure 37 Key Buying Criteria for End-users
6 Clinical Trials Market, by Phase
6.1 Introduction
Table 16 Clinical Trials Industry, by Phase, 2021-2028 (USD Million)
Figure 38 Drugs in Pipeline, by Development Phase, 2021 Vs. 2022
6.2 Phase Iii Clinical Trials
6.2.1 Rising Prices of Phase Iii Trials to Drive Demand for Cost-Effective Clinical Research Services
Table 17 Overview of Drugs That Have Completed Phase Iii (2021-2022)
Table 18 Market, by Region, 2021-2028 (USD Million)
Table 19 North America: Market, by Country, 2021-2028 (USD Million)
Table 20 Europe: Market, by Country, 2021-2028 (USD Million)
Table 21 Asia-Pacific: Market, by Country, 2021-2028 (USD Million)
6.3 Phase Ii Clinical Trials
6.3.1 Long Duration of Phase Ii Studies to Provide Growth Opportunities for Cros
Table 22 Overview of Drugs That Have Completed Phase Ii (2021-2022)
Table 23 Market, by Region, 2021-2028 (USD Million)
Table 24 North America: Market, by Country, 2021-2028 (USD Million)
Table 25 Europe: Market, by Country, 2021-2028 (USD Million)
Table 26 Asia-Pacific: Market, by Country, 2021-2028 (USD Million)
6.4 Phase Iv Clinical Trials
6.4.1 Rising Number of Clinical Research Entities Providing Post-Marketing Surveillance to Drive Market
Table 27 Overview of Drugs That Have Completed Phase Iv (2021-2022)
Table 28 Market, by Region, 2021-2028 (USD Million)
Table 29 North America: Market, by Country, 2021-2028 (USD Million)
Table 30 Europe: Market, by Country, 2021-2028 (USD Million)
Table 31 Asia-Pacific: Market, by Country, 2021-2028 (USD Million)
6.5 Phase I Clinical Trials
6.5.1 Robust Pipeline of Pharmaceutical Companies to Boost Market
Table 32 Overview of Drugs in Phase I Studies (2021-2022)
Table 33 Market, by Region, 2021-2028 (USD Million)
Table 34 North America: Market, by Country, 2021-2028 (USD Million)
Table 35 Europe: Market, by Country, 2021-2028 (USD Million)
Table 36 Asia-Pacific: Market, by Country, 2021-2028 (USD Million)
7 Clinical Trials Market, by Service Type
7.1 Introduction
Table 37 Clinical Trials Industry, by Service Type, 2021-2028 (USD Million)
7.2 Laboratory Services
7.2.1 Rising Importance of Lab Services and Need to Ensure Regulatory Compliance to Drive Market
Table 38 Laboratory Services Offered by Prominent Players
Table 39 Laboratory Services Market, by Region, 2021-2028 (USD Million)
Table 40 North America: Laboratory Services Market, by Country, 2021-2028 (USD Million)
Table 41 Europe: Laboratory Services Market, by Country, 2021-2028 (USD Million)
Table 42 Asia-Pacific: Laboratory Services Market, by Country, 2021-2028 (USD Million)
7.3 Bioanalytical Testing Services
Table 43 Bioanalytical Testing Services Market, by Type, 2021-2028 (USD Million)
Table 44 Bioanalytical Testing Services Market, by Region, 2021-2028 (USD Million)
Table 45 North America: Bioanalytical Testing Services Market, by Country, 2021-2028 (USD Million)
Table 46 Europe: Bioanalytical Testing Services Market, by Country, 2021-2028 (USD Million)
Table 47 Asia-Pacific: Bioanalytical Testing Services Market, by Country, 2021-2028 (USD Million)
7.3.1 Cell-Based Assays
7.3.1.1 Rising Number of Providers Offering Customized Assay Testing to Boost Growth
Table 48 Cell-Based Assays Market, by Region, 2021-2028 (USD Million)
Table 49 North America: Cell-Based Assays Market, by Country, 2021-2028 (USD Million)
Table 50 Europe: Cell-Based Assays Market, by Country, 2021-2028 (USD Million)
Table 51 Asia-Pacific: Cell-Based Assays Market, by Country, 2021-2028 (USD Million)
7.3.2 Virology Testing
7.3.2.1 Rising Uptake of in Vitro Virology Assays to Develop Antiviral Pharmaceuticals to Favor Market
Table 52 Virology Testing Market, by Region, 2021-2028 (USD Million)
Table 53 North America: Virology Testing Market, by Country, 2021-2028 (USD Million)
Table 54 Europe: Virology Testing Market, by Country, 2021-2028 (USD Million)
Table 55 Asia-Pacific: Virology Testing Market, by Country, 2021-2028 (USD Million)
7.3.3 Serology, Immunogenicity, and Neutralizing Antibodies
7.3.3.1 High Demand for Immunogenicity and Neutralizing Antibody Assays in Biosimilar Development to Drive Market
Table 56 Serology, Immunogenicity, and Neutralizing Antibodies Market, by Region, 2021-2028 (USD Million)
Table 57 North America: Serology, Immunogenicity, and Neutralizing Antibodies Market, by Country, 2021-2028 (USD Million)
Table 58 Europe: Serology, Immunogenicity, and Neutralizing Antibodies Market, by Country, 2021-2028 (USD Million)
Table 59 Asia-Pacific: Serology, Immunogenicity, and Neutralizing Antibodies Market, by Country, 2021-2028 (USD Million)
7.3.4 Pk/Pd Testing
7.3.4.1 Growing Importance of Drug Pharmacokinetics to Boost Demand
Table 60 Pk/Pd Testing Market, by Region, 2021-2028 (USD Million)
Table 61 North America: Pk/Pd Testing Market, by Country, 2021-2028 (USD Million)
Table 62 Europe: Pk/Pd Testing Market, by Country, 2021-2028 (USD Million)
Table 63 Asia-Pacific: Pk/Pd Testing Market, by Country, 2021-2028 (USD Million)
7.3.5 Method Development, Optimization, and Validation
7.3.5.1 Biopharma Industry Preference for Outsourcing Method Validation to Drive Market
Table 64 Method Development, Optimization, and Validation Market, by Region, 2021-2028 (USD Million)
Table 65 North America: Method Development, Optimization, and Validation Market, by Country, 2021-2028 (USD Million)
Table 66 Europe: Method Development, Optimization, and Validation Market, by Country, 2021-2028 (USD Million)
Table 67 Asia-Pacific: Method Development, Optimization, and Validation Market, by Country, 2021-2028 (USD Million)
7.3.6 Biomarker Testing
7.3.6.1 Growing Emphasis on Biomarker Testing to Develop Personalized Medicines and Cdx to Augment Market
Table 68 Biomarker Testing Market, by Region, 2021-2028 (USD Million)
Table 69 North America: Biomarker Testing Market, by Country, 2021-2028 (USD Million)
Table 70 Europe: Biomarker Testing Market, by Country, 2021-2028 (USD Million)
Table 71 Asia-Pacific: Biomarker Testing Services Market, by Country, 2021-2028 (USD Million)
7.3.7 Other Bioanalytical Testing Services
Table 72 Other Bioanalytical Testing Services Market, by Region, 2021-2028 (USD Million)
Table 73 North America: Other Bioanalytical Testing Services Market, by Country, 2021-2028 (USD Million)
Table 74 Europe: Other Bioanalytical Testing Services Market, by Country, 2021-2028 (USD Million)
Table 75 Asia-Pacific: Other Bioanalytical Testing Services Market, by Country, 2021-2028 (USD Million)
7.4 Decentralized Clinical Trial Services
7.4.1 High Demand for Dct Attributed to Advancements in Digital Health Technologies
Table 76 Decentralized Clinical Trial Services Market, by Region, 2021-2028 (USD Million)
Table 77 North America: Decentralized Clinical Trial Services Market, by Country, 2021-2028 (USD Million)
Table 78 Europe: Decentralized Clinical Trial Services Market, by Country, 2021-2028 (USD Million)
Table 79 Asia-Pacific: Decentralized Clinical Trial Services Market, by Country, 2021-2028 (USD Million)
7.5 Patient Recruitment Services
7.5.1 Growing Need for Patient-Centric Clinical Trial Services to Support Demand
Table 80 Overview of Patient Recruitment Service Providers
Table 81 Patient Recruitment Services Market, by Region, 2021-2028 (USD Million)
Table 82 North America: Patient Recruitment Services Market, by Country, 2021-2028 (USD Million)
Table 83 Europe: Patient Recruitment Services Market, by Country, 2021-2028 (USD Million)
Table 84 Asia-Pacific: Patient Recruitment Services Market, by Country, 2021-2028 (USD Million)
7.6 Site Identification Services
7.6.1 Introduction of Digital Technologies Enabling Data Variability to Drive Market
Table 85 Site Identification Services Market, by Region, 2021-2028 (USD Million)
Table 86 North America: Site Identification Services Market, by Country, 2021-2028 (USD Million)
Table 87 Europe: Site Identification Services Market, by Country, 2021-2028 (USD Million)
Table 88 Asia-Pacific: Site Identification Services Market, by Country, 2021-2028 (USD Million)
7.7 Analytical Testing Services
7.7.1 Increasing Demand for Analytical Testing in Drug Development to Drive Market
Table 89 Analytical Testing Services Market, by Region, 2021-2028 (USD Million)
Table 90 North America: Analytical Testing Services Market, by Country, 2021-2028 (USD Million)
Table 91 Europe: Analytical Testing Services Market, by Country, 2021-2028 (USD Million)
Table 92 Asia-Pacific: Analytical Testing Services Market, by Country, 2021-2028 (USD Million)
7.8 Supply & Logistics Services
7.8.1 Usage of Advanced Data Analytics in Clinical Trial Supply Management to Drive Market
Table 93 Supply & Logistics Services Market, by Region, 2021-2028 (USD Million)
Table 94 North America: Supply & Logistics Services Market, by Country, 2021-2028 (USD Million)
Table 95 Europe: Supply & Logistics Services Market, by Country, 2021-2028 (USD Million)
Table 96 Asia-Pacific: Supply & Logistics Services Market, by Country, 2021-2028 (USD Million)
7.9 Protocol Designing Services
7.9.1 Need for Planning Support to Boost Demand for Protocol Design
Table 97 Protocol Designing Services Market, by Region, 2021-2028 (USD Million)
Table 98 North America: Protocol Designing Services Market, by Country, 2021-2028 (USD Million)
Table 99 Europe: Protocol Designing Services Market, by Country, 2021-2028 (USD Million)
Table 100 Asia-Pacific: Protocol Designing Services Market, by Country, 2021-2028 (USD Million)
7.10 Data Management Services
7.10.1 Data Management Services to Record Highest CAGR Over Forecast Period
Table 101 Data Management Services Offered by Prominent Players
Table 102 Data Management Services Market, by Region, 2021-2028 (USD Million)
Table 103 North America: Data Management Services Market, by Country, 2021-2028 (USD Million)
Table 104 Europe: Data Management Services Market, by Country, 2021-2028 (USD Million)
Table 105 Asia-Pacific: Data Management Services Market, by Country, 2021-2028 (USD Million)
7.11 Medical Device Testing Services
7.11.1 Increasing Medical Device R&D to Drive Market
Table 106 Medical Device Testing Services Market, by Region, 2021-2028 (USD Million)
Table 107 North America: Medical Device Testing Services Market, by Country, 2021-2028 (USD Million)
Table 108 Europe: Medical Device Testing Services Market, by Country, 2021-2028 (USD Million)
Table 109 Asia-Pacific: Medical Device Testing Services Market, by Country, 2021-2028 (USD Million)
7.12 Other Services
Table 110 Other Clinical Trial Services Market, by Region, 2021-2028 (USD Million)
Table 111 North America: Other Clinical Trial Services Market, by Country, 2021-2028 (USD Million)
Table 112 Europe: Other Clinical Trial Services Market, by Country, 2021-2028 (USD Million)
Table 113 Asia-Pacific: Other Clinical Trial Services Market, by Country, 2021-2028 (USD Million)
8 Clinical Trials Market, by Therapeutic Area
8.1 Introduction
Table 114 Clinical Trials Industry, by Therapeutic Area, 2021-2028 (USD Million)
8.2 Oncology
8.2.1 Oncology to Dominate Market due to Rising Incidence of Cancer
Figure 39 Us Cancer Incidence in Males, by Type (2022)
Figure 40 Us Cancer Incidence in Females, by Type (2022)
Figure 41 Oncology Clinical Trials Globally, 2010-2021 (Thousand)
Table 115 New Fda-Approved Oncology Drugs (2023)
Table 116 Clinical Trials Industry for Oncology, by Region, 2021-2028 (USD Million)
Table 117 North America: Market for Oncology, by Country, 2021-2028 (USD Million)
Table 118 Europe: Market for Oncology, by Country, 2021-2028 (USD Million)
Table 119 Asia-Pacific: Market for Oncology, by Country, 2021-2028 (USD Million)
8.3 Infectious Diseases
8.3.1 Infectious Diseases to Hold Second-Largest Market Share, by Therapeutic Area
Table 120 Market for Infectious Diseases, by Region, 2021-2028 (USD Million)
Table 121 North America: Market for Infectious Diseases, by Country, 2021-2028 (USD Million)
Table 122 Europe: Market for Infectious Diseases, by Country, 2021-2028 (USD Million)
Table 123 Asia-Pacific: Market for Infectious Diseases, by Country, 2021-2028 (USD Million)
8.4 Neurology
8.4.1 Increasing Investments in R&D and Grants for Neurological Disorders to Drive Market
Table 124 List of Pipeline Drugs for Neurological Disorders (2020)
Table 125 Market for Neurology, by Region, 2021-2028 (USD Million)
Table 126 North America: Market for Neurology, by Country, 2021-2028 (USD Million)
Table 127 Europe: Market for Neurology, by Country, 2021-2028 (USD Million)
Table 128 Asia-Pacific: Market for Neurology, by Country, 2021-2028 (USD Million)
8.5 Metabolic Disorders
8.5.1 Rising Diabetes Incidence to Drive Market
Figure 42 Increase in Diabetes Cases, 2000-2045 (Million Individuals)
Table 129 Clinical Trials Industry for Metabolic Disorders, by Region, 2021-2028 (USD Million)
Table 130 North America: Market for Metabolic Disorders, by Country, 2021-2028 (USD Million)
Table 131 Europe: Market for Metabolic Disorders, by Country, 2021-2028 (USD Million)
Table 132 Asia-Pacific: Market for Metabolic Disorders, by Country, 2021-2028 (USD Million)
8.6 Immunology
8.6.1 Growing Pipeline of Immunological Drugs to Boost Outsourcing
Table 133 List of Pipeline Drugs for Immunological Disorders (2022)
Table 134 Market for Immunology, by Region, 2021-2028 (USD Million)
Table 135 North America: Market for Immunology, by Country, 2021-2028 (USD Million)
Table 136 Europe: Market for Immunology, by Country, 2021-2028 (USD Million)
Table 137 Asia-Pacific: Market for Immunology, by Country, 2021-2028 (USD Million)
8.7 Cardiology
8.7.1 High Mortality Rates to Drive Development of New Treatments
Table 138 List of Pipeline Drugs for Cardiovascular Diseases (2023)
Table 139 Market for Cardiology, by Region, 2021-2028 (USD Million)
Table 140 North America: Market for Cardiology, by Country, 2021-2028 (USD Million)
Table 141 Europe: Market for Cardiology, by Country, 2021-2028 (USD Million)
Table 142 Asia-Pacific: Market for Cardiology, by Country, 2021-2028 (USD Million)
8.8 Genetic Diseases
8.8.1 Government Support for Rare & Genetic Disease Therapeutics to Boost Market
Table 143 Clinical Trials Industry for Genetic Diseases, by Region, 2021-2028 (USD Million)
Table 144 North America: Market for Genetic Diseases, by Country, 2021-2028 (USD Million)
Table 145 Europe: Market for Genetic Diseases, by Country, 2021-2028 (USD Million)
Table 146 Asia-Pacific: Market for Genetic Diseases, by Country, 2021-2028 (USD Million)
8.9 Women's Health
8.9.1 Rising Prevalence of Chronic Disorders in Women to Propel Demand
Table 147 Market for Women's Health, by Region, 2021-2028 (USD Million)
Table 148 North America: Market for Women's Health, by Country, 2021-2028 (USD Million)
Table 149 Europe: Market for Women's Health, by Country, 2021-2028 (USD Million)
Table 150 Asia-Pacific: Market for Women's Health, by Country, 2021-2028 (USD Million)
8.10 Other Therapeutic Areas
Table 151 List of Pipeline Drugs for Skin Diseases (2022)
Table 152 Market for Other Therapeutic Areas, by Region, 2021-2028 (USD Million)
Table 153 North America: Market for Other Therapeutic Areas, by Country, 2021-2028 (USD Million)
Table 154 Europe: Market for Other Therapeutic Areas, by Country, 2021-2028 (USD Million)
Table 155 Asia-Pacific: Market for Other Therapeutic Areas, by Country, 2021-2028 (USD Million)
9 Clinical Trials Market, by Application
9.1 Introduction
Table 156 Clinical Trials Industry, by Application, 2021-2028 (USD Million)
9.2 Small Molecules
9.2.1 Investments in Small-Molecule Development to Support Growth
Table 157 Market for Small Molecules, by Region, 2021-2028 (USD Million)
Table 158 North America: Market for Small Molecules, by Country, 2021-2028 (USD Million)
Table 159 Europe: Market for Small Molecules, by Country, 2021-2028 (USD Million)
Table 160 Asia-Pacific: Market for Small Molecules, by Country, 2021-2028 (USD Million)
9.3 Monoclonal Antibodies
9.3.1 Growing Government Support for Monoclonal Antibodies to Drive Market
Table 161 Market for Monoclonal Antibodies, by Region, 2021-2028 (USD Million)
Table 162 North America: Market for Monoclonal Antibodies, by Country, 2021-2028 (USD Million)
Table 163 Europe: Market for Monoclonal Antibodies, by Country, 2021-2028 (USD Million)
Table 164 Asia-Pacific: Market for Monoclonal Antibodies, by Country, 2021-2028 (USD Million)
9.4 Vaccines
9.4.1 Shifting Industry Focus Toward Chronic Disease Treatment to Slow Market Growth
Figure 43 Number of Clinical Trials for Vaccines (2020-2023)
Table 165 Market for Vaccines, by Region, 2021-2028 (USD Million)
Table 166 North America: Market for Vaccines, by Country, 2021-2028 (USD Million)
Table 167 Europe: Market for Vaccines, by Country, 2021-2028 (USD Million)
Table 168 Asia-Pacific: Market for Vaccines, by Country, 2021-2028 (USD Million)
9.5 Cell & Gene Therapy
9.5.1 Rising Trend of Outsourcing to Favor Market Growth
Figure 44 Ongoing Clinical Trials, by Phase and Therapeutic Approach (2022)
Table 169 Market for Cell & Gene Therapy, by Region, 2021-2028 (USD Million)
Table 170 North America: Market for Cell & Gene Therapy, by Country, 2021-2028 (USD Million)
Table 171 Europe: Market for Cell & Gene Therapy, by Country, 2021-2028 (USD Million)
Table 172 Asia-Pacific: Market for Cell & Gene Therapy, by Country, 2021-2028 (USD Million)
9.6 Other Applications
Table 173 Market for Other Applications, by Region, 2021-2028 (USD Million)
Table 174 North America: Market for Other Applications, by Country, 2021-2028 (USD Million)
Table 175 Europe: Market for Other Applications, by Country, 2021-2028 (USD Million)
Table 176 Asia-Pacific: Market for Other Applications, by Country, 2021-2028 (USD Million)
10 Clinical Trials Market, by Region
10.1 Introduction
Table 177 Clinical Trials Industry, by Region, 2021-2028 (USD Million)
10.2 North America
10.2.1 North America: Recession Impact
Figure 45 North America: Clinical Trials Market Snapshot
Table 178 North America: Market, by Country, 2021-2028 (USD Million)
Table 179 North America: Market, by Phase, 2021-2028 (USD Million)
Table 180 North America: Market, by Service Type, 2021-2028 (USD Million)
Table 181 North America: Bioanalytical Testing Services Market, by Type, 2021-2028 (USD Million)
Table 182 North America: Market, by Therapeutic Area, 2021-2028 (USD Million)
Table 183 North America: Clinical Trials Market, by Application, 2021-2028 (USD Million)
10.2.2 US
10.2.2.1 Us to Hold Largest Share of North American Market Over Forecast Period
Figure 46 Distribution of R&D Companies, by Country/Region (2022 Vs. 2023): Us to Hold Dominant Share
Table 184 US: Clinical Trials Market, by Phase, 2021-2028 (USD Million)
Table 185 US: Market, by Service Type, 2021-2028 (USD Million)
Table 186 US: Bioanalytical Testing Services Market, by Type, 2021-2028 (USD Million)
Table 187 US: Market, by Therapeutic Area, 2021-2028 (USD Million)
Table 188 US: Clinical Trials Industry, by Application, 2021-2028 (USD Million)
10.2.3 Canada
10.2.3.1 Increasing Clinical Trial Activity to Support Market Growth
Table 189 Canada: Clinical Trials Market, by Phase, 2021-2028 (USD Million)
Table 190 Canada: Market, by Service Type, 2021-2028 (USD Million)
Table 191 Canada: Bioanalytical Testing Services Market, by Type, 2021-2028 (USD Million)
Table 192 Canada: Market, by Therapeutic Area, 2021-2028 (USD Million)
Table 193 Canada: Clinical Trials Industry, by Application, 2021-2028 (USD Million)
10.3 Europe
10.3.1 Europe: Recession Impact
Figure 47 Pharmaceutical Industry R&D Spending in European Countries, 2021 (USD Million)
Table 194 Europe: Clinical Trials Market, by Country, 2021-2028 (USD Million)
Table 195 Europe: Market, by Phase, 2021-2028 (USD Million)
Table 196 Europe: Market, by Service Type, 2021-2028 (USD Million)
Table 197 Europe: Bioanalytical Testing Services Market, by Type, 2021-2028 (USD Million)
Table 198 Europe: Market, by Therapeutic Area, 2021-2028 (USD Million)
Table 199 Europe: Market, by Application, 2021-2028 (USD Million)
10.3.2 Germany
10.3.2.1 Germany to Dominate Clinical Trials Market in Europe
Table 200 Germany: Market, by Phase, 2021-2028 (USD Million)
Table 201 Germany: Market, by Service Type, 2021-2028 (USD Million)
Table 202 Germany: Bioanalytical Testing Services Market, by Type, 2021-2028 (USD Million)
Table 203 Germany: Market, by Therapeutic Area, 2021-2028 (USD Million)
Table 204 Germany: Clinical Trials Industry, by Application, 2021-2028 (USD Million)
10.3.3 UK
10.3.3.1 Investments in Drug Discovery Services to Support Market Growth
Figure 48 UK: Pharmaceutical R&D Expenditure, 2018-2021 (USD Million)
Table 205 UK: Clinical Trials Market, by Phase, 2021-2028 (USD Million)
Table 206 UK: Market, by Service Type, 2021-2028 (USD Million)
Table 207 UK: Bioanalytical Testing Services Market, by Type, 2021-2028 (USD Million)
Table 208 UK: Market, by Therapeutic Area, 2021-2028 (USD Million)
Table 209 UK: Clinical Trials Market, by Application, 2021-2028 (USD Million)
10.3.4 France
10.3.4.1 High Number of Oncology Clinical Trials to Drive Market
Table 210 France: Clinical Trials Market, by Phase, 2021-2028 (USD Million)
Table 211 France: Market, by Service Type, 2021-2028 (USD Million)
Table 212 France: Bioanalytical Testing Services Market, by Type, 2021-2028 (USD Million)
Table 213 France: Market, by Therapeutic Area, 2021-2028 (USD Million)
Table 214 France: Clinical Trials Industry, by Application, 2021-2028 (USD Million)
10.3.5 Italy
10.3.5.1 Low Drug Approval Times and Growing Number of Clinical Trials to Boost Market
Table 215 Italy: Clinical Trials Market, by Phase, 2021-2028 (USD Million)
Table 216 Italy: Market, by Service Type, 2021-2028 (USD Million)
Table 217 Italy: Bioanalytical Testing Services Market, by Type, 2021-2028 (USD Million)
Table 218 Italy: Market, by Therapeutic Area, 2021-2028 (USD Million)
Table 219 Italy: Clinical Trials Industry, by Application, 2021-2028 (USD Million)
10.3.6 Spain
10.3.6.1 Rising R&D Expenditure to Drive Market
Figure 49 Spain: Pharmaceutical R&D Expenditure, 2018-2021 (USD Million)
Table 220 Spain: Clinical Trials Market, by Phase, 2021-2028 (USD Million)
Table 221 Spain: Market, by Service Type, 2021-2028 (USD Million)
Table 222 Spain: Bioanalytical Testing Services Market, by Type, 2021-2028 (USD Million)
Table 223 Spain: Market, by Therapeutic Area, 2021-2028 (USD Million)
Table 224 Spain: Clinical Trials Industry, by Application, 2021-2028 (USD Million)
10.3.7 Rest of Europe
Table 225 Rest of Europe: Clinical Trials Market, by Phase, 2021-2028 (USD Million)
Table 226 Rest of Europe: Market, by Service Type, 2021-2028 (USD Million)
Table 227 Rest of Europe: Bioanalytical Testing Services Market, by Type, 2021-2028 (USD Million)
Table 228 Rest of Europe: Market, by Therapeutic Area, 2021-2028 (USD Million)
Table 229 Rest of Europe: Market, by Application, 2021-2028 (USD Million)
10.4 Asia-Pacific
10.4.1 Asia-Pacific: Recession Impact
Figure 50 Asia-Pacific: Clinical Trials Market Snapshot
Table 230 Asia-Pacific: Market, by Country, 2021-2028 (USD Million)
Table 231 Asia-Pacific: Market, by Phase, 2021-2028 (USD Million)
Table 232 Asia-Pacific: Market, by Service Type, 2021-2028 (USD Million)
Table 233 Asia-Pacific: Bioanalytical Testing Services Market, by Type, 2021-2028 (USD Million)
Table 234 Asia-Pacific: Market, by Therapeutic Area, 2021-2028 (USD Million)
Table 235 Asia-Pacific: Clinical Trials Industry, by Application, 2021-2028 (USD Million)
10.4.2 China
10.4.2.1 China to Dominate APAC Clinical Trial Market
Table 236 China: Clinical Trials Market, by Phase, 2021-2028 (USD Million)
Table 237 China: Market, by Service Type, 2021-2028 (USD Million)
Table 238 China: Bioanalytical Testing Services Market, by Type, 2021-2028 (USD Million)
Table 239 China: Market, by Therapeutic Area, 2021-2028 (USD Million)
Table 240 China: Clinical Trials Market, by Application, 2021-2028 (USD Million)
10.4.3 Japan
10.4.3.1 Strong Focus on R&D Activities to Support Growth
Figure 51 Top Therapeutic Areas of Industry-Sponsored Clinical Trials in Japan
Table 241 Japan: Clinical Trials Market, by Phase, 2021-2028 (USD Million)
Table 242 Japan: Market, by Service Type, 2021-2028 (USD Million)
Table 243 Japan: Bioanalytical Testing Services Market, by Type, 2021-2028 (USD Million)
Table 244 Japan: Market, by Therapeutic Area, 2021-2028 (USD Million)
Table 245 Japan: Clinical Trials Industry, by Application, 2021-2028 (USD Million)
10.4.4 India
10.4.4.1 Growing Pharmaceutical Industry to Drive Market
Table 246 India: Clinical Trials Market, by Phase, 2021-2028 (USD Million)
Table 247 India: Market, by Service Type, 2021-2028 (USD Million)
Table 248 India: Bioanalytical Testing Services Market, by Type, 2021-2028 (USD Million)
Table 249 India: Market, by Therapeutic Area, 2021-2028 (USD Million)
Table 250 India: Market, by Application, 2021-2028 (USD Million)
10.4.5 Rest of Asia-Pacific
Figure 52 Breakdown of Studies in Malaysia, by Intervention, 2021
Table 251 Rest of Asia-Pacific: Clinical Trials Market, by Phase, 2021-2028 (USD Million)
Table 252 Rest of Asia-Pacific: Market, by Service Type, 2021-2028 (USD Million)
Table 253 Rest of Asia-Pacific: Bioanalytical Testing Services Market, by Type, 2021-2028 (USD Million)
Table 254 Rest of Asia-Pacific: Market, by Therapeutic Area, 2021-2028 (USD Million)
Table 255 Rest of Asia-Pacific: Clinical Trials Industry, by Application, 2021-2028 (USD Million)
10.5 Latin America
10.5.1 Rising Pharma & Biotech R&D to Boost Market Growth
10.5.2 Latin America: Recession Impact
Table 256 Latin America: Clinical Trials Market, by Phase, 2021-2028 (USD Million)
Table 257 Latin America: Market, by Service Type, 2021-2028 (USD Million)
Table 258 Latin America: Bioanalytical Testing Services Market, by Type, 2021-2028 (USD Million)
Table 259 Latin America: Market, by Therapeutic Area, 2021-2028 (USD Million)
Table 260 Latin America: Clinical Trials Market, by Application, 2021-2028 (USD Million)
10.6 Middle East & Africa
10.6.1 Growing Pharmaceutical Industry to Drive Market
10.6.2 Middle East & Africa: Recession Impact
Table 261 Middle East & Africa: Clinical Trials Market, by Phase, 2021-2028 (USD Million)
Table 262 Middle East & Africa: Market, by Service Type, 2021-2028 (USD Million)
Table 263 Middle East & Africa: Bioanalytical Testing Services Market, by Type, 2021-2028 (USD Million)
Table 264 Middle East & Africa: Market, by Therapeutic Area, 2021-2028 (USD Million)
Table 265 Middle East & Africa: Clinical Trials Industry, by Application, 2021-2028 (USD Million)
11 Competitive Landscape
11.1 Introduction
11.2 Key Player Strategies/Right to Win
Figure 53 Overview of Strategies Deployed by Key Market Players
11.3 Revenue Share Analysis
Figure 54 Revenue Analysis of Key Players, 2020-2022
11.4 Market Share Analysis
Figure 55 Market Share Analysis of Key Players, 2022
Table 266 Clinical Trials Market: Degree of Competition
11.5 Company Evaluation Matrix, 2022
Figure 56 Clinical Trials Market: Company Evaluation Matrix, 2022
11.5.1 Stars
11.5.2 Emerging Leaders
11.5.3 Pervasive Players
11.5.4 Participants
11.6 Company Footprint Analysis of Top 25 Players
11.6.1 Industry Footprint (25 Companies)
Table 267 Industry Footprint Analysis: Key Players
11.6.2 Service Footprint (25 Companies)
Table 268 Service Footprint Analysis: Key Players
11.6.3 Regional Footprint (25 Companies)
Table 269 Regional Footprint Analysis: Key Players
11.7 Company Evaluation Matrix for Start-Ups/Smes, 2022
Figure 57 Clinical Trials Market: Company Evaluation Matrix for Start-Ups/Smes, 2022
11.7.1 Progressive Companies
11.7.2 Starting Blocks
11.7.3 Responsive Companies
11.7.4 Dynamic Companies
11.8 Competitive Benchmarking of Start-Ups/Smes
Table 270 Clinical Trials Industry: Detailed List of Key Start-Ups/Smes
Table 271 Service Footprint Analysis: Start-Up/Smes
Table 272 Regional Footprint Analysis: Start-Ups/Smes
11.9 Competitive Scenario and Trends
11.9.1 Service Launches
Table 273 Clinical Trials Industry: Service Launches (2020-2023)
11.9.2 Deals
Table 274 Clinical Trials Market: Deals (2020-2023)
11.9.3 Other Developments
Table 275 Clinical Trials Market: Other Developments (2020-2023)
12 Company Profiles
(Business Overview, Products Offered, Recent Developments, Analyst's View Right to Win, Strategic Choices Made, Weaknesses and Competitive Threats) *
12.1 Key Companies
12.1.1 Thermo Fisher Scientific, Inc.
Table 276 Thermo Fisher Scientific, Inc.: Business Overview
Figure 58 Thermo Fisher Scientific, Inc.: Company Snapshot
12.1.2 Iqvia, Inc.
Table 277 Iqvia, Inc.: Business Overview
Figure 59 Iqvia, Inc.: Company Snapshot
12.1.3 Icon plc
Table 278 Icon plc: Business Overview
Figure 60 Icon plc: Company Snapshot
12.1.4 Laboratory Corporation of America Holdings
Table 279 Laboratory Corporation of America Holdings: Business Overview
Figure 61 Laboratory Corporation of America Holdings: Company Snapshot
12.1.5 Syneos Health
Table 280 Syneos Health: Business Overview
Figure 62 Syneos Health: Company Snapshot
12.1.6 Wuxi Apptec
Table 281 Wuxi Apptec: Business Overview
Figure 63 Wuxi Apptec: Company Snapshot
12.1.7 Charles River Laboratories
Table 282 Charles River Laboratories: Business Overview
Figure 64 Charles River Laboratories: Company Snapshot
12.1.8 Parexel International Corporation
Table 283 Parexel International Corporation: Business Overview
12.1.9 Fortrea, Inc.
Table 284 Fortrea, Inc.: Business Overview
Figure 65 Fortrea, Inc.: Company Snapshot
12.1.10 Medpace
Table 285 Medpace: Business Overview
Figure 66 Medpace: Company Snapshot
12.1.11 Sgs
Table 286 Sgs: Business Overview
Figure 67 Sgs: Company Snapshot
12.1.12 Frontage Labs
Table 287 Frontage Labs: Business Overview
Figure 68 Frontage Labs: Company Snapshot
12.1.13 Acm Global Laboratories
Table 288 Acm Global Laboratories: Business Overview
12.1.14 Advanced Clinical
Table 289 Advanced Clinical: Business Overview
12.1.15 Psi
Table 290 Psi: Business Overview
12.1.16 Bioagile
Table 291 Bioagile: Business Overview
12.2 Other Players
12.2.1 Clinical Trial Service
12.2.2 Worldwide Clinical Trials
12.2.3 Pepgra
12.2.4 Cti Clinical Trial and Consulting Services
12.2.5 Dove Quality Solutions
12.2.6 Firma Clinical Research
12.2.7 Celerion
12.2.8 Novotech
12.2.9 Linical Americas
*Details on Business Overview, Products Offered, Recent Developments, Analyst's View, Right to Win, Strategic Choices Made, Weaknesses and Competitive Threats Might Not be Captured in Case of Unlisted Companies.
13 Appendix
13.1 Discussion Guide
13.2 Knowledgestore: The Subscription Portal
13.3 Customization Options