Speak directly to the analyst to clarify any post sales queries you may have.
Human Immunodeficiency Virus (HIV) is a viral infection that attacks the body's immune system and does not have effective care treatment across the world. It leads to acquired immunodeficiency syndrome (AIDS). Globally, more than 38.4 million people are living with HIV infection, among which Africa accounted for the higher patient population. HIV is one of the leading causes of death in the world. The Joint United Nations Programme on HIV/AIDS and WHO are two major health organizations working towards increasing awareness among people about HIV, HIV infection, and associated preventive measures. These two organizations aim to reduce around 90% HIV burden of the world by 2030. Based on that, several countries joined these initiatives to improve their national HIV status. HIV self-testing (HIVST) is one of the major strategies addressed by WHO and UNIAIDS to overcome the HIV burden. These steps lead to an increase in demand for HIVST that directly impact the HIV rapid test kits market.
MARKET TRENDS & OPPORTUNITIES
Raise of Next-Generation HIV Test Technology
The rise of fourth-generation rapid test kits has become more popular in recent years. This technology is more accurate and reliable for screening the acute HIV presence in the sample. The 4th generation HIV test easily detects HIV in lesser time and more accurately than other generation technologies. Such factors allow individuals to get a cure sooner and potentially prevent the spread of infection to others. One of the leading HIV rapid test kit market players, OraSure technologies, got approval for a home test kit known as “OraQuick In-Home HIV Test,” which gives results in 20 to 40 minutes.Increasing HIV Self-Testing Associated Government Policies
The UNAID and WHO are working together towards HIV prevention. Several strategic implementations are implemented worldwide to increase HIV self-testing (HIVST) and to understand the actual burden of HIV/AIDS globally. Eastern and Southern Africa, and Western and Central Europe & North America are the regions that implemented several HIVST policies that deliver HIV rapid test kits market growth opportunities. Due to the increasing acceptance of HIVST, the knowledge and awareness about HIV increased among the people, resulting in many people looking for easy HIV testing.New Paradigm of Digital Support In HIV Self-Testing
The COVID-19 pandemic led to several service disruptions in the supply of HIV diagnoses and treatments, which led to falling short of the UNAIDS 2030 target. However, the COVID-19 pandemic accelerated the application of digital tools in health services. The incorporation of digital tools gave a new paradigm in HIV care management. The application of digital tools in HIV management is increasing that expected to fuel the HIV rapid test kits market. According to The Lancet research, the adoption and innovation of digital HIV self-testing tools have been increasing recently, and website-based innovations are becoming more popular.Increasing Targeted Patient Population
Around 15,000,000 people acquired HIV in 2020. In 2022, more than 38 million people were expected to live with HIV. This further results in increasing HIV preventive measures, which include increasing the number of people undergoing HIV tests through self-test kits. In high-infection regions such as Africa, Europe, and Asian countries, the demand for HIV testing is increasing year by year, which drives the application rate of HIV rapid test kits and propels market growth.Increasing Awareness about HIV Self-Testing
Most countries are making significant progress in reducing the HIV burden with UNAIDS and WHO. Globally, around 95% of people living with HIV are expected to be aware of their HIV status by 2025. Further, the increasing prevalence of HIV, the awareness among HIV-infected and non-infected people in developed and developing countries is increasing rapidly. In developed countries, HIV infection awareness increased through various government and health authorities’ programs working as support to improve knowledge about HIV self-testing solutions. The increasing access to HIV rapid test kits through health authorities and vendor activities and the acceptance of self-testing kits for HIV management are increasing simultaneously, fueling the HIV rapid test kits market growth.SEGMENTATION INSIGHTS
INSIGHTS BY TECHNOLOGY
The global HIV rapid test kits market based on technology is segmented into lateral flow immunoassay and immunofixation. The lateral flow immunoassay segment accounted for a 78.25% market share in 2021. A higher number of HIV rapid test kit products are available and approved by various health authorities in the market and are developed by the lateral flow immunoassay technology. This technology delivers several advantaged and accurate results in HIV rapid testing. One of the major reasons for increasing preference towards the usage of HIV rapid test kits is their immediate results. The quicker results will help healthcare professionals to make treatment plans in the market. Quicker diagnosis of the disease will help the healthcare providers to safeguard the patients and to take enough preventive measures to reduce the spread of the disease in the market.Segmentation by Technology
- Lateral Flow Immunoassay
- Immunofiltration
INSIGHTS BY SAMPLE TYPE
The blood sample type segment accounted for a higher 63.46% market share in the global HIV rapid test kits market and dominated the other segments. Blood sample-based HIV rapid test kits are highly recommended by medical professionals due to their potential to carry HIV antibodies in high proportion. In HIV testing, blood samples (plasma, serum, DBS) are highly preferred for HIV rapid testing. Blood samples carry higher concentrations of HIV antibodies than oral (saliva) samples. These sample-based HIV rapid testing found antibodies using a drop of blood from the fingertip. In addition to the individuals, healthcare settings also use HIV rapid test kits that drive blood sample segmental growth. In addition, HIV rapid test kits are distributed to patients through clinical laboratories, research centers, and community-based organizations.Segmentation by Sample Type
- Blood
- Oral Fluid
- Urine
INSIGHTS BY DISTRIBUTION CHANNELS
The offline segment dominates the distribution channels and accounts for a 66.70% global HIV rapid test kits market share. Offline sales channels included medical shops, pharmacies, hospitals, clinics, associated pharmacies, and pharma retailers. Offline sales channels are the first point of resource for HIV testing in LMICs. In most developing countries, people choose medical pharmacies as the first care point to buy HIV rapid test kits. Easy access and availability with the convenience of buyers are the major factors that drive major sales through offline channels. In low- and middle-income countries, drug shops are critical in healthcare provisions, where they are often the first point of care access for medical solutions. Drug shops are the best alternative to conventional facility-based HIV testing and counseling, and offline stores offer significant advantages in those regions for HIV rapid test kits. Consumers avoid a longer wait and potential clinical staff and associated charges.Segmentation by Distribution Channels
- Offline
- Online
GEOGRAPHICAL ANALYSIS
North America dominates the global HIV rapid test kits market accounting for a 31.06% market share. The factors that propel the market growth in the region are the higher HIV prevalence and the increasing awareness among the key patient populations. The government support and funding for HIV management as preventive measures drive the region's significant market growth. More than 3.8 million people in North America are estimated for HIV in 2022. The awareness about HIV self-testing is significantly high in the US, while Canada is showing progressive growth for HIV self-testing.The Europe HIV rapid test kits market was valued at USD 158.77 million in 2022. It is estimated that the rate and number of new diagnoses of HIV infection are continuously increasing in East Europe and are growing rapidly, though from a much lower base in Central Europe region.
Segmentation by Geography
- North America
- US
- Canada
- Europe
- France
- Italy
- Ukraine
- Portugal
- Russia
- APAC
- China
- India
- Indonesia
- Thailand
- Vietnam
- Latin America
- Brazil
- Mexico
- Columbia
- Middle East & Africa
- South Africa
- Mozambique
- Kenya
- Nigeria
- Tanzania
VENDOR LANDSCAPE
The global HIV rapid test kits market has many global, regional, and domestic players that offer a comprehensive product portfolio of HIV rapid test kits. Some global market players have acquired high industry penetration with a broader distribution network and business strategies. Also, some companies collaborate with WHO and UNAIDS to increase the increasing rate of HIV self-testing by supporting HIV rapid test kits. In 2022, WHO partnered with the Clinton Health Access Initiative (CHAI), Wondfo Biotech Company, and MedAccess. This partnership makes available HIV-self-test kits for only USD 1 in the public sector of LMICs. This is expected to increase the high industry competition among leading market players. The inorganic growth strategies of leading market players create entry barriers for many small and emerging players.Key Company Profiles
- Abbott
- Bio-Rad Laboratories
- BIOMÉRIEUX
- Chembio Diagnostics
- OraSure Technologies
Other Prominent Vendors
- Atomo Diagnostics
- AccuBioTech
- bioLytical Laboratories
- Biosynex
- Cupid Limited
- DIALAB
- HUMAN
- INTEC
- J. Mitra & Co
- Meril Life Sciences
- MP Biomedicals
- Medsource Ozone Biomedicals
- Nanjing Synthgene Medical Technology
- Premier Medical
- SD Biosensor
- KHB (Shanghai Kehua Bio-Engineering co., Ltd.)
- Türklab A.S.
- Trinity Biotech
- Wantai BioPharma
- Wondfo
KEY QUESTIONS ANSWERED:
1. How big is the HIV rapid test kits market?2. What is the growth rate of the HIV rapid test kits market?
3. Which region holds the most significant global HIV rapid test kits market share?
4. What are the growing trends in the HIV rapid test kits market?
5. Which are the key companies in the global HIV rapid test kits market?
Table of Contents
1 Research Methodology2 Research Objectives
3 Research Process
4 Scope & Coverage
4.1 Market Definition
4.1.1 Inclusions
4.1.2 Exclusions
4.1.3 Market Estimation Caveats
4.2 Base Year
4.3 Scope of the Study
4.3.1 Market by Technology
4.3.2 Market by Sample Type
4.3.3 Market by Distribution Channels
4.3.4 Market Segmentation by Geography
5 Report Assumptions & Caveats
5.1 Key Caveats
5.2 Currency Conversion
5.3 Market Derivation
6 Market at a Glance
7 Premium Insights
7.1 Overview
8 Introduction
8.1 Overview
9 Market Opportunities & Trends
9.1 Introduction of Next Generation Hiv Rapid Test Kits
9.2 Increase in Hiv Self-Testing by Government Bodies
9.3 Digital Support for Hiv Self-Testing
10 Market Growth Enablers
10.1 Increasing Hiv Patient Population
10.2 Increased Resources Availability and Funding for Hiv Management
10.3 Increased Awareness of Hiv Self-Testing
11 Market Restraints
11.1 Increasing Incidences of False Negatives
11.2 Limitations & Challenges Associated with Hiv Testing
11.3 Low Accessiblity & Lack of Awareness for Hivst
12 Market Landscape
12.1 Market Overview
12.1.1 Insights by Technology
12.1.2 Insights by Sample Type
12.1.3 Insights by Distribution Channels
12.1.4 Insights by Geography
12.2 Market Size & Forecast
12.3 Five Forces Analysis
12.3.1 Threat of New Entrants
12.3.2 Bargaining Power of Suppliers
12.3.3 Bargaining Power of Buyers
12.3.4 Threat of Substitutes
12.3.5 Competitive Rivalry
13 Technology
13.1 Market Snapshot & Growth Engine
13.2 Market Overview
13.3 Lateral Flow Immunoassay
13.3.1 Market Overview
13.3.2 Market Size & Forecast
13.3.3 Market by Geography
13.4 Immunofiltration
13.4.1 Market Overview
13.4.2 Market Size & Forecast
13.4.3 Market by Geography
14 Sample Type
14.1 Market Snapshot & Growth Engine
14.2 Market Overview
14.3 Blood
14.3.1 Market Overview
14.3.2 Market Size & Forecast
14.3.3 Market by Geography
14.4 Oral Fluid
14.4.1 Market Overview
14.4.2 Market Size & Forecast
14.4.3 Market by Geography
14.5 Urine
14.5.1 Market Overview
14.5.2 Market Size & Forecast
14.5.3 Market by Geography
15 Distribution Channel
15.1 Market Snapshot & Growth Engine
15.2 Market Overview
15.3 Offline
15.3.1 Market Overview
15.3.2 Market Size & Forecast
15.3.3 Market by Geography
15.4 Online
15.4.1 Market Overview
15.4.2 Market Size & Forecast
15.4.3 Market by Geography
16 Geography
16.1 Market Snapshot & Growth Engine
16.2 Geographic Overview
17 North America
17.1 Market Overview
17.2 Market Size & Forecast
17.3 Technology
17.3.1 Market Size & Forecast
17.4 Sample Type
17.4.1 Market Size & Forecast
17.5 Distribution Channels
17.5.1 Market Size & Forecast
17.6 Key Countries
17.6.1 Us: Market Size & Forecast
17.6.2 Canada: Market Size & Forecast
18 APAC
18.1 Market Overview
18.2 Market Size & Forecast
18.3 Technology
18.3.1 Market Size & Forecast
18.4 Sample Type
18.4.1 Market Size & Forecast
18.5 Distribution Channels
18.5.1 Market Size & Forecast
18.6 Key Countries
18.6.1 China: Market Size & Forecast
18.6.2 India: Market Size & Forecast
18.6.3 Indonesia: Market Size & Forecast
18.6.4 Thailand: Market Size & Forecast
18.6.5 Vietnam: Market Size & Forecast
19 Middle East & Africa
19.1 Market Overview
19.2 Market Size & Forecast
19.3 Technology
19.3.1 Market Size & Forecast
19.4 Sample Type
19.4.1 Market Size & Forecast
19.5 Distribution Channels
19.5.1 Market Size & Forecast
19.6 Key Countries
19.6.1 South Africa: Market Size & Forecast
19.6.2 Mozambique: Market Size & Forecast
19.6.3 Kenya: Market Size & Forecast
19.6.4 Nigeria: Market Size & Forecast
19.6.5 Tanzania: Market Size & Forecast
20 Europe
20.1 Market Overview
20.2 Market Size & Forecast
20.3 Technology
20.3.1 Market Size & Forecast
20.4 Sample Type
20.4.1 Market Size & Forecast
20.5 Distribution Channels
20.5.1 Market Size & Forecast
20.6 Key Countries
20.6.1 France: Market Size & Forecast
20.6.2 Italy: Market Size & Forecast
20.6.3 Ukraine: Market Size & Forecast
20.6.4 Portugal: Market Size & Forecast
20.6.5 Russia: Market Size & Forecast
21 Latin America
21.1 Market Overview
21.2 Market Size & Forecast
21.3 Technology
21.3.1 Market Size & Forecast
21.4 Sample Type
21.4.1 Market Size & Forecast
21.5 Distribution Channels
21.5.1 Market Size & Forecast
21.6 Key Countries
21.6.1 Brazil: Market Size & Forecast
21.6.2 Mexico: Market Size & Forecast
21.6.3 Colombia: Market Size & Forecast
22 Competitive Landscape
22.1 Competition Overview
22.2 Market Share Analysis
22.2.1 Abbott
22.2.2 Bio-Rad Laboratories
22.2.3 Biomérieux
22.2.4 Chembio Diagnostics
22.2.5 Orasure Technologies
23 Key Company Profiles
23.1 Abbott
23.1.1 Business Overview
23.1.2 Product Offerings
23.1.3 Key Strategies
23.1.4 Key Strengths
23.1.5 Key Opportunities
23.2 Bio-Rad Laboratories
23.2.1 Business Overview
23.2.2 Product Offerings
23.2.3 Key Strategies
23.2.4 Key Strengths
23.2.5 Key Opportunities
23.3 Biomérieux
23.3.1 Business Overview
23.3.2 Product Offerings
23.3.3 Key Strategies
23.3.4 Key Strengths
23.3.5 Key Opportunities
23.4 Chembio Diagnostics
23.4.1 Business Overview
23.4.2 Product Offerings
23.4.3 Key Strategies
23.4.4 Key Strengths
23.4.5 Key Opportunities
23.5 Orasure Technologies
23.5.1 Business Overview
23.5.2 Product Offerings
23.5.3 Key Strategies
23.5.4 Key Strengths
23.5.5 Key Opportunities
24 Other Prominent Vendors
24.1 Atomo Diagnostics
24.1.1 Business Overview
24.1.2 Product Offerings
24.2 Accubiotech
24.2.1 Business Overview
24.2.2 Product Offerings
24.3 Biolytical Laboratories
24.3.1 Business Overview
24.3.2 Product Offerings
24.4 Biosynex
24.4.1 Business Overview
24.4.2 Product Offerings
24.5 Cupid Limited
24.5.1 Business Overview
24.5.2 Product Offerings
24.6 Human
24.6.1 Business Overview
24.6.2 Product Offerings
24.7 Intec
24.7.1 Business Overview
24.7.2 Product Offerings
24.8 J. Mitra & Co
24.8.1 Business Overview
24.8.2 Product Offerings
24.9 Meril Life Sciences
24.9.1 Business Overview
24.9.2 Product Offerings
24.10 Mp Biomedicals
24.10.1 Business Overview
24.10.2 Product Offerings
24.11 Medsource Ozone Biomedicals
24.11.1 Business Overview
24.11.2 Product Offerings
24.12 Nanjing Synthgene Medical Technology
24.12.1 Business Overview
24.12.2 Product Offerings
24.13 Premier Medical
24.13.1 Business Overview
24.13.2 Product Offerings
24.14 Sd Biosensor
24.14.1 Business Overview
24.14.2 Product Offerings
24.15 Khb (Shanghai Kehua Bio-Engineering)
24.15.1 Business Overview
24.15.2 Product Offerings
24.16 Türklab A.S.
24.16.1 Business Overview
24.16.2 Product Offerings
24.17 Trinity Biotech
24.17.1 Business Overview
24.17.2 Product Offerings
24.18 Wantai Biopharma
24.18.1 Business Overview
24.18.2 Product Offerings
24.19 Wondfo
24.19.1 Business Overview
24.19.2 Product Offerings
25 Report Summary
25.1 Key Takeaways
25.2 Strategic Recommendations
26 Quantitative Summary
26.1 Market by Geography
26.2 Market by Technology
26.3 Market by Sample Type
26.4 Market by Distribution Channels
26.5 Technology: Market by Geography
26.5.1 Lateral Flow Immunoassay: Market by Geography
26.5.2 Immunofiltration: Market by Geography
26.6 Sample Type: Market by Geography
26.6.1 Blood Sample Type: Market by Geography
26.6.2 Oral Fluid Sample Type: Market by Geography
26.6.3 Urine Sample Type: Market by Geography
26.7 Distribution Channels: Market by Geography
26.7.1 Offline Distribution Channels: Market by Geography
26.7.2 Online Distribution Channels: Market by Geography
27 Appendix
27.1 Abbreviations
Companies Mentioned
- Abbott
- Bio-Rad Laboratories
- BIOMÉRIEUX
- Chembio Diagnostics
- OraSure Technologies
- Atomo Diagnostics
- AccuBioTech
- bioLytical Laboratories
- Biosynex
- Cupid Limited
- DIALAB
- HUMAN
- INTEC
- J. Mitra & Co
- Meril Life Sciences
- MP Biomedicals
- Medsource Ozone Biomedicals
- Nanjing Synthgene Medical Technology
- Premier Medical
- SD Biosensor
- KHB (Shanghai Kehua Bio-Engineering co., Ltd.)
- Türklab A.S.
- Trinity Biotech
- Wantai BioPharma
- Wondfo
Methodology
Our research comprises a mix of primary and secondary research. The secondary research sources that are typically referred to include, but are not limited to, company websites, annual reports, financial reports, company pipeline charts, broker reports, investor presentations and SEC filings, journals and conferences, internal proprietary databases, news articles, press releases, and webcasts specific to the companies operating in any given market.
Primary research involves email interactions with the industry participants across major geographies. The participants who typically take part in such a process include, but are not limited to, CEOs, VPs, business development managers, market intelligence managers, and national sales managers. We primarily rely on internal research work and internal databases that we have populated over the years. We cross-verify our secondary research findings with the primary respondents participating in the study.

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 266 |
| Published | February 2023 |
| Forecast Period | 2022 - 2028 |
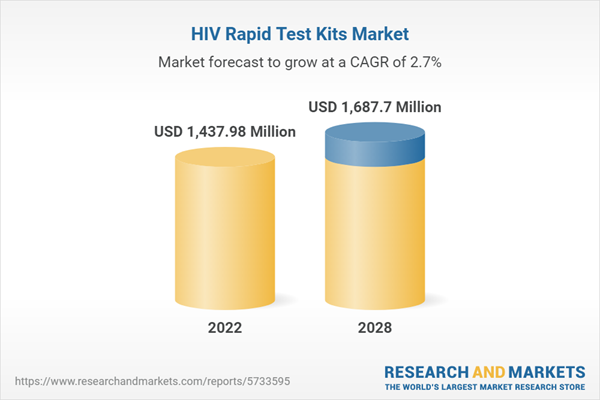
| Estimated Market Value ( USD | $ 1437.98 Million |
| Forecasted Market Value ( USD | $ 1687.7 Million |
| Compound Annual Growth Rate | 2.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |







![In-Vitro Diagnostics Market by Offering (Kits, Software), Technology (Immunoassay, Molecular Diagnostics [PCR, NGS, Microarray], Rapid Tests, Biochemistry), Application (Infectious Diseases, Oncology), Diagnostic Approach (Lab, POC) - Global Forecast to 2030 - Product Image](http://www.researchandmarkets.com/product_images/12002/12002788_60px_jpg/invitro_diagnostics_market.jpg)
