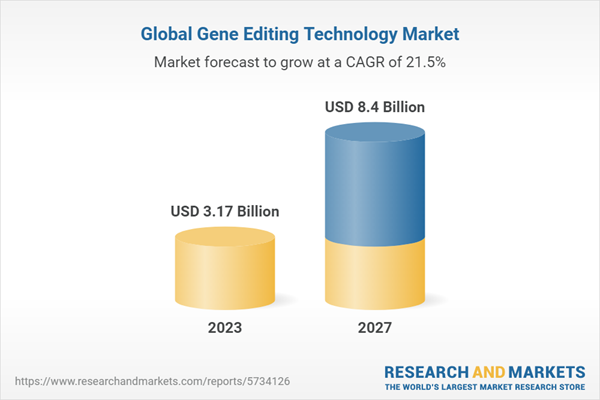
According to the publisher, the global gene editing technology market size is expected to record a CAGR of 21.5% during 2023-2027 to reach US$8.400 billion by 2027, increasing from US$3.171 billion in 2023. Over the last five years, the sector has recorded a CAGR of 27.5% to reach US$2.488 billion in 2022.
Gene editing technology has emerged as one of the fastest-growing areas in the global healthcare sector. With the emerging technology showing immense potential to cure some of the world’s most feared diseases, including cancer, gene-editing technology can revolutionize the global healthcare landscape. Global gene editing market forecast reveals that the revolutionary CRISPR gene editing technology has sped its way from the lab to the clinical field within a span of a single decade.
Notably, the CRISPR gene editing technology has turned out to be the founding stone of many innovative biotech start-ups, while also creating tens of billions of dollars in value. Looking at the potential of gene editing technology, big pharmaceutical players such as Bayer, Pfizer, and Regeneron have all shown interest in the space. Over the years, these firms have forged alliances with pioneers in the sector, with the hope to find treatment for life-limiting and life-threatening diseases, including cancer.
Going forward, gene editing market trends show that these big corporations in the healthcare sector are expected to further continue with the trend of forging alliances with emerging start-ups, while also seeking buyout opportunities proactively, thereby allowing them to acquire tools to improve current treatments. Furthermore, big pharmaceutical giants are sitting on huge piles of cash and many of them are also in need to expand their product pipelines, as many of the patents would expire at the end of the ongoing decade. Consequently, every large pharmaceutical firm is expected to have some sort of gene editing capabilities, either through strategic collaborations or the acquisition of smaller firms.
Some researchers have already started to make progress in the segment; however, global gene editing market analysis shows that it is expected to blow up over the next few years. Furthermore, research in the space will also expand the number of diseases that can be treated using the CRISPR gene editing technology.
Along with the healthcare sector, gene-editing technology is also expanding its roots in the agricultural market around the world. The need to ensure food security for their people has led several governments in enhancing their regulatory environment, thereby supporting the usage of gene-editing technology in the agricultural sector. Even European regulators, who have long opposed the implementation of gene editing technology for the production of crops, are also re-evaluating their viewpoint on the technology, to meet the growing food demand among people across Europe.
Climate change and an unstable political environment have had a severe impact on the global production and availability of crops. Gene editing has shown the potential to develop climate-resistant and higher-yield crops. Consequently, the agricultural sector is another area of focus for biotech firms that are looking to drive growth in the gene editing market over the next few years.
Looking at the growth potential of the sector, venture capital firms have invested billions of dollars in the space over the last few years, and the trend is expected to further continue from the short to medium-term perspective. As new areas of gene editing application emerge and breakthrough products are developed, the interest among venture capital firms, globally, is expected to balloon over the next three to four years. This will keep driving innovation and competitive landscape from the short to medium-term perspective, thereby supporting the gene editing market growth.
This report presents an in-depth analysis of the current gene editing market scenario, with an emphasis on the future trajectory of potential opportunities in the gene editing industry. In addition to a combination of exhaustive secondary research along with primary research, the publisher's research methodology leverages a proprietary predictive analytics platform to provide unbiased business intelligence.
It provides a detailed analysis of gene editing market dynamics, covering clinical trials, patent data, financial deals, and company profiling details. It details market opportunities and risks across key segments - by technology, products, delivery method, disease, and applications. It also details market dynamics across various end-use sectors to assess emerging opportunities.
In addition, this report provides analyst commentary on key trends, drivers, strategies, innovations, and regulations in gene editing technology market.
Gene editing technology has emerged as one of the fastest-growing areas in the global healthcare sector. With the emerging technology showing immense potential to cure some of the world’s most feared diseases, including cancer, gene-editing technology can revolutionize the global healthcare landscape. Global gene editing market forecast reveals that the revolutionary CRISPR gene editing technology has sped its way from the lab to the clinical field within a span of a single decade.
Notably, the CRISPR gene editing technology has turned out to be the founding stone of many innovative biotech start-ups, while also creating tens of billions of dollars in value. Looking at the potential of gene editing technology, big pharmaceutical players such as Bayer, Pfizer, and Regeneron have all shown interest in the space. Over the years, these firms have forged alliances with pioneers in the sector, with the hope to find treatment for life-limiting and life-threatening diseases, including cancer.
Going forward, gene editing market trends show that these big corporations in the healthcare sector are expected to further continue with the trend of forging alliances with emerging start-ups, while also seeking buyout opportunities proactively, thereby allowing them to acquire tools to improve current treatments. Furthermore, big pharmaceutical giants are sitting on huge piles of cash and many of them are also in need to expand their product pipelines, as many of the patents would expire at the end of the ongoing decade. Consequently, every large pharmaceutical firm is expected to have some sort of gene editing capabilities, either through strategic collaborations or the acquisition of smaller firms.
Some researchers have already started to make progress in the segment; however, global gene editing market analysis shows that it is expected to blow up over the next few years. Furthermore, research in the space will also expand the number of diseases that can be treated using the CRISPR gene editing technology.
- The Somatic Cell Genome Editing (SCGE) Program, at the National Institutes of Health (NIH) has given 24 new funds to researchers in the United States and Canada to assist them. Over four years, this collection of SCGE Program awards will total about $89 million. Assuming finances are available, this raises the overall number of projects sponsored to 45 and the total financing to around $190 million over six years.
Along with the healthcare sector, gene-editing technology is also expanding its roots in the agricultural market around the world. The need to ensure food security for their people has led several governments in enhancing their regulatory environment, thereby supporting the usage of gene-editing technology in the agricultural sector. Even European regulators, who have long opposed the implementation of gene editing technology for the production of crops, are also re-evaluating their viewpoint on the technology, to meet the growing food demand among people across Europe.
Climate change and an unstable political environment have had a severe impact on the global production and availability of crops. Gene editing has shown the potential to develop climate-resistant and higher-yield crops. Consequently, the agricultural sector is another area of focus for biotech firms that are looking to drive growth in the gene editing market over the next few years.
Looking at the growth potential of the sector, venture capital firms have invested billions of dollars in the space over the last few years, and the trend is expected to further continue from the short to medium-term perspective. As new areas of gene editing application emerge and breakthrough products are developed, the interest among venture capital firms, globally, is expected to balloon over the next three to four years. This will keep driving innovation and competitive landscape from the short to medium-term perspective, thereby supporting the gene editing market growth.
This report presents an in-depth analysis of the current gene editing market scenario, with an emphasis on the future trajectory of potential opportunities in the gene editing industry. In addition to a combination of exhaustive secondary research along with primary research, the publisher's research methodology leverages a proprietary predictive analytics platform to provide unbiased business intelligence.
It provides a detailed analysis of gene editing market dynamics, covering clinical trials, patent data, financial deals, and company profiling details. It details market opportunities and risks across key segments - by technology, products, delivery method, disease, and applications. It also details market dynamics across various end-use sectors to assess emerging opportunities.
In addition, this report provides analyst commentary on key trends, drivers, strategies, innovations, and regulations in gene editing technology market.
This title provides global and regional insights along with data-centric analysis for the following 6 regions and 21 countries:
- Global
- North America
- United States
- Canada
- Mexico
- Europe
- France
- Germany
- United Kingdom
- Italy
- Switzerland
- Asia-Pacific
- China
- Japan
- Australia
- India
- South Korea
- Latin America
- Brazil
- Argentina
- Colombia
- MEA
- Israel
- South Africa
- Turkey
- UAE
- Saudi Arabia
Scope
Insights and data in the report has been segmented under the following six modules:Module 1: Global Gene Editing Funding and Investments Outlook
This module provides insights and data related to gene editing partnerships and investment (research and development, mergers and acquisitions, product development, commercialization, licensing, and manufacturing) and allows a peek into the futuristic trends of gene editing technology investment area.Module 2: Global Gene Editing Clinical Trial Data Assessment
This module evaluates the data available from clinical trials conducted for various CRISPR, ZFNs, TALENs, and meganucleases-based therapies, representing a bird’s eye view of emerging market dynamics and risks in the gene editing sector.Module 3: Global Gene Editing Patent Data Analysis
This module presents an exhaustive study of gene editing patent analytics (CRISPR, ZFN, TALENs, and meganucleases) at various levels, including strategic research planning as well as analyzing their potential applications.Module 4: Competitive Landscape
This module provides detailed information along with a scoring matrix of key companies related to the gene editing industry, including their historical performance and the latest developments.Module 5: Global Gene Editing Market Size and Forecast
This module provides projections on the market's development during the years 2018-2027 after the profound evaluation of market dynamics at the deeper segmentation level. This report also provides an in-depth, data-centric analysis of the global gene editing market at regional and country levels:By Technology
- CRISPR CAS 9
- TALENs
- ZFN
- Others (Meganucleases)
By Products
- Kits & Enzymes (By CRISPR, TALENs, ZFN, Meganucleases)
- Cell lines & Antibodies (By CRISPR, TALENs, ZFN, Meganucleases)
- Plasmid & Controls (By CRISPR, TALENs, ZFN, Meganucleases)
By Delivery Method
- Ex-vivo
- In-vivo
By Disease/Disorders
- Cancer
- Blood Disorders (beta-thalassemia and sickle cell disease)
- Blindness
- Respiratory Disease (COVID-19 & Cystic fibrosis)
- Others (AIDS, Huntington’s disease, Muscular dystrophy)
By Application
- Drug Development
- Diagnostics
- Plant Gene Editing
- Others (Animal Gene Editing)
By End-User
- Pharmaceutical and Biopharmaceutical Companies
- Biotechnology Companies
- Contract Research Organisations
- Academic Institutes and Research Centres
By Regions
- North America
- U. S
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Germany
- Italy
- Switzerland
- Asia-Pacific
- China
- Japan
- Australia
- India
- South Korea
- MEA region
- Israel
- South Africa
- Turkey
- U.A. E
- Saudi Arabia
- Latin America
- Brazil
- Argentina
Key Companies Covered
- bluebird bio
- Beam Therapeutics
- Caribou Biosciences
- Cellectis
- Allogene Therapeutics
- Intellia Therapeutics
- Editas Medicine
- Verve Therapeutics
- AstraZeneca
- Merck KGaA
- Thermo Fisher Scientific
Module 6 Regulatory Outlook
This module contains a detailed understanding of the ethical, legal, and social implications of gene editing technology.Reasons to buy
- Gene Editing Market Dynamics: Understand market opportunities and key trends along with forecast (2023-2027). Understand market dynamics across various segments such as by technology, products, delivery method, disease/disorders, application, geographical region, and company.
- Competitive Landscape Analysis: Market position and snapshot of key players in the development of therapies based on CRISPR, meganucleases, TALENs, and ZFN. Each profile includes a brief overview of the company, information on its therapeutic pipeline, financial data (where available), the most recent events, and a knowledgeable prediction for the future.
- Clinical Trial Analysis: Insights into Opportunity by a thorough evaluation of clinical trials involving various ZFNs, TALENs, and meganucleases-based therapies that have been completed or are still ongoing, using parameters such as trial status, trial registration year, an indication of the target disease, trial phase, patient enrolment, and sponsor/collaborator type.
- Develop Market-Specific Strategies: Identify growth segments based on a thorough analysis of the CRISPR, ZFN, TALENs, and meganucleases patents that have been applied for or granted based on the patent type, the issuing agency, and patent offices involved, the Cooperative Patent Classification (CPC) symbols, the firm type, significant industry and non-industry participants (based on many patents), and discrete patent assignees.
- Financial Deals Insights: Get Insights and information about various gene editing partnerships that have been developed, including those for research and development (R&D), clinical trials, mergers and acquisitions, product development, commercialization, licencing, and manufacturing. A complete analysis of the various investments made in companies that specialise in developing drugs using CRISPR, ZFNs, TALENs, and meganucleases.
Table of Contents
Chapter 1 About this Report
Chapter 2 Gene Editing Market Dynamics
Chapter 3 Global Gene Editing Technology Discovery and Clinical Pipeline Market Attractiveness
Chapter 4 Gene Editing Technology Financial Deals & Alliances: Analysis of Emerging Market Dynamics & Outlook, 2018-2027
Chapter 5 Gene Editing Technology Patent Analysis & Outlook
Chapter 6 Clinical Trial Analysis & Outlook, 2017-2027
Chapter 7 Gene Editing Technology Competitive Landscape Analysis: Key Company Profiles and Strategic Initiatives
Chapter 8 Gene Editing Technology Market Size and Forecast, 2018-2027
Companies Mentioned
- bluebirdbio
- Beam Therapeutics
- Caribou Biosciences
- Cellectis
- Allogene Therapeutics
- Intellia Therapeutics Inc.
- Editas Medicine
- Verve Therapeutics
- AstraZeneca
- Merck KGaA
- Thermo Fisher Scientific Inc.
Methodology

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 1300 |
| Published | August 2023 |
| Forecast Period | 2023 - 2027 |
| Estimated Market Value ( USD | $ 3.17 Billion |
| Forecasted Market Value ( USD | $ 8.4 Billion |
| Compound Annual Growth Rate | 21.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |