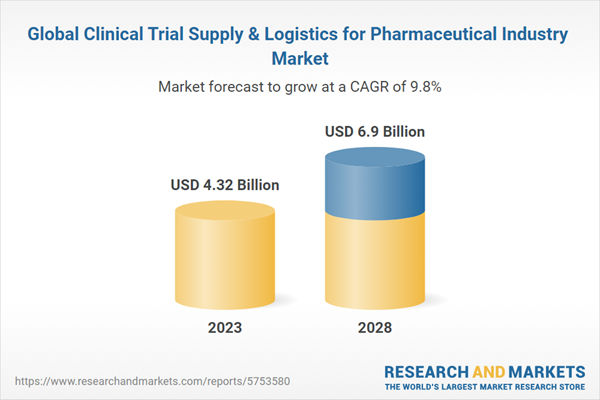
The Global Clinical Trial Supply & Logistics for Pharmaceutical Industry Market is estimated to be USD 4.32 Bn in 2023 and is expected to reach USD 6.9 Bn by 2028 growing at a CAGR of 9.81%.
As the market dynamics impact the supply and demand curves, decision-makers aim to determine the best way to use various financial tools to stem various strategies for speeding growth and reducing the risks.
Market Dynamics
Market dynamics are forces that impact the prices and behaviors of the stakeholders. These forces create pricing signals which result from the changes in the supply and demand curves for a given product or service. Forces of Market Dynamics may be related to macroeconomic and microeconomic factors. There are dynamic market forces other than price, demand, and supply. Human emotions can also drive decisions, influence the market, and create price signals.As the market dynamics impact the supply and demand curves, decision-makers aim to determine the best way to use various financial tools to stem various strategies for speeding growth and reducing the risks.
Market Segmentations
- The Global Clinical Trial Supply & Logistics for Pharmaceutical Industry Market is segmented based on Phase, Sector, Therapeutic Area, and Geography.
- By Phase, the market is classified into BA/BE Studies, Phase I, Phase II, Phase III, and Phase IV.
- By Sector, the market is classified into Clinical Trial Logistics and Distributions, Clinical Trial Manufacturing Services, and Clinical Trial Supply Chain Management.
- By Therapeutic Area, the market is classified into Blood Disorders, Digestive System Diseases, Infectious and Immune System Diseases, Neurological and Mental Disorders, and Oncology.
- By Geography, the market is classified into Americas, Europe, Middle East & Africa, and Asia-Pacific.
Company Profiles
The report provides a detailed analysis of the competitors in the market. It covers the financial performance analysis for publicly listed companies in the market. The report also offers detailed information on the companies' recent development and competitive scenario. Some of the companies covered in this report are Almac Group, AmerisourceBergen Corp., Bilcare Ltd., Biocair International Ltd., Catalent, Inc., Clinigen Group PLC, DHL International GmbH by Deutsche Post Ag, Eurofins Scientific SE, etc.Countries Studied
- America (Argentina, Brazil, Canada, Chile, Colombia, Mexico, Peru, United States, Rest of Americas)
- Europe (Austria, Belgium, Denmark, Finland, France, Germany, Italy, Netherlands, Norway, Poland, Russia, Spain, Sweden, Switzerland, United Kingdom, Rest of Europe)
- Middle East and Africa (Egypt, Israel, Qatar, Saudi Arabia, South Africa, United Arab Emirates, Rest of MEA)
- Asia-Pacific (Australia, Bangladesh, China, India, Indonesia, Japan, Malaysia, Philippines, Singapore, South Korea, Sri Lanka, Thailand, Taiwan, Rest of Asia-Pacific)
Competitive Quadrant
The report includes Competitive Quadrant, a proprietary tool to analyze and evaluate the position of companies based on their Industry Position score and Market Performance score. The tool uses various factors for categorizing the players into four categories. Some of these factors considered for analysis are financial performance over the last 3 years, growth strategies, innovation score, new product launches, investments, growth in market share, etc.Ansoff Analysis
- The report presents a detailed Ansoff matrix analysis for the Global Clinical Trial Supply & Logistics for Pharmaceutical Industry Market. Ansoff Matrix, also known as the Product/Market Expansion Grid, is a strategic tool used to design strategies for the growth of the company. The matrix can be used to evaluate approaches in four strategies viz. Market Development, Market Penetration, Product Development, and Diversification. The matrix is also used for risk analysis to understand the risk involved with each approach.
- The publisher analyses the Global Clinical Trial Supply & Logistics for Pharmaceutical Industry Market using the Ansoff Matrix to provide the best approaches a company can take to improve its market position.
- Based on the SWOT analysis conducted on the industry and industry players, the publisher has devised suitable strategies for market growth.
Why buy this report?
- The report offers a comprehensive evaluation of the Global Clinical Trial Supply & Logistics for the Pharmaceutical Industry Market. The report includes in-depth qualitative analysis, verifiable data from authentic sources, and projections about market size. The projections are calculated using proven research methodologies.
- The report has been compiled through extensive primary and secondary research. The primary research is done through interviews, surveys, and observation of renowned personnel in the industry.
- The report includes an in-depth market analysis using Porter's 5 forces model and the Ansoff Matrix. In addition, the impact of COVID-19 and impact of economic slowdown & impending recession on the market are also featured in the report.
- The report also includes the regulatory scenario in the industry, which will help you make a well-informed decision. The report discusses major regulatory bodies and major rules and regulations imposed on this sector across various geographies.
- The report also contains the competitive analysis using Positioning Quadrants, the Proprietary competitive positioning tool.
Report Highlights:
- A complete analysis of the market, including parent industry
- Important market dynamics and trends
- Market segmentation
- Historical, current, and projected size of the market based on value and volume
- Market shares and strategies of key players
- Recommendations to companies for strengthening their foothold in the market
Table of Contents
1 Report Description
2 Research Methodology
3 Executive Summary
4 Market Dynamics
5 Market Analysis
6 Global Clinical Trial Supply & Logistics for Pharmaceutical Industry Market, By Phase
7 Global Clinical Trial Supply & Logistics for Pharmaceutical Industry Market, By Sector
8 Global Clinical Trial Supply & Logistics for Pharmaceutical Industry Market, By Therapeutic Area
9 Americas' Clinical Trial Supply & Logistics for Pharmaceutical Industry Market
10 Europe's Clinical Trial Supply & Logistics for Pharmaceutical Industry Market
11 Middle East and Africa's Clinical Trial Supply & Logistics for Pharmaceutical Industry Market
12 APAC's Clinical Trial Supply & Logistics for Pharmaceutical Industry Market
13 Competitive Landscape
14 Company Profiles
15 Appendix
Companies Mentioned
- Almac Group
- AmerisourceBergen Corp.
- Bilcare Ltd
- Biocair International Ltd
- Catalent, Inc.
- Clinigen Group PLC
- DHL International GmbH (Deutsche Post Ag)
- Eurofins Scientific SE
- FedEx Corp.
- ICON PLC
- Infosys Ltd
- KLIFO A/S
- Lonza Group
- Marken Ltd.
- Movianto
- Parexel International Corp.
- PCI Pharma Services
- Seveillar Clinical Trial Supplies Pvt. Ltd.
- SIRO Clinpharm Pvt. Ltd.
- Thermo Fisher Scientific, Inc.
- UDG Healthcare PLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 191 |
| Published | February 2024 |
| Forecast Period | 2023 - 2028 |
| Estimated Market Value ( USD | $ 4.32 Billion |
| Forecasted Market Value ( USD | $ 6.9 Billion |
| Compound Annual Growth Rate | 9.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |