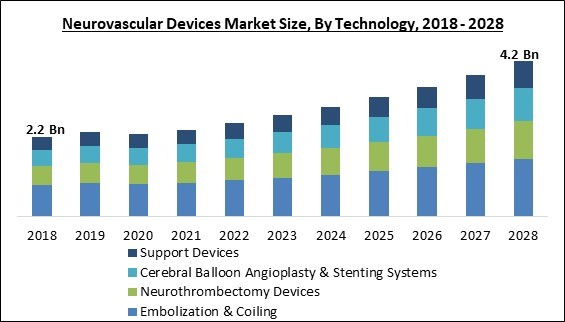
The Global Neurovascular Devices Market size is expected to reach $4.2 billion by 2028, rising at a market growth of 8.8% CAGR during the forecast period.
Neurovascular devices are used to treat neurovascular conditions. Stents, coils, and clips are examples of neurovascular devices used to treat conditions like brain aneurysms. Similar devices are used to remove blood clots from the brain's arteries and veins, which can result in life-threatening disorders like stroke. This equipment includes suction and aspiration devices. Furthermore, ischemic stroke, stenosis, aneurysm, arteriovenous malformation, and fistulas are a few other neurovascular disorders that can be treated with the help of neurovascular devices.
Central nervous system or brain vascular disorders are diagnosed and treated with interventional neurology technologies. Interventional neurology includes angiography, endovascular operations, fluoroscopy,and catheter-based procedures. For example, several neurological illnesses, such as arteriovenous malformations,cerebral aneurysm,arteriovenous fistula, intracranial stenosis, and vasculitis, can be diagnosed with catheter angiography, one of the earliest in-vivo brain vascular imaging procedures.
Due to the decreased danger and trauma involved with these treatments, minimally invasive procedures are becoming increasingly common. In addition, smaller incisions reduce postoperative pain and speed up healing, resulting in high adoption of these operations and increasing R&D in this area. To bring cutting-edge minimally invasive surgical equipment to market, several important firms are investing in R&D. One of the minimally invasive procedures that doctors highly suggest is the endovascular coiling used to treat cerebral aneurysms.
During this surgery, a microcatheter is placed via the aneurysm-containing artery in the groin area. Platinum coils are released to encourage aneurysm coagulation and stop blood from entering other parts of the brain. The considerable volume of neurovascular devices consumed, which has contributed to the market expansion, is primarily due to the fast-expanding patient population for the target disorders across the key markets.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Abbott Laboratories, Medtronic PLC, Stryker Corporation, Johnson & Johnson, Terumo Corporation, Merit Medical Systems, Inc., MicroPort Scientific Corporation, Medikit Co. Ltd., and W. L. Gore & Associates, Inc.
Neurovascular devices are used to treat neurovascular conditions. Stents, coils, and clips are examples of neurovascular devices used to treat conditions like brain aneurysms. Similar devices are used to remove blood clots from the brain's arteries and veins, which can result in life-threatening disorders like stroke. This equipment includes suction and aspiration devices. Furthermore, ischemic stroke, stenosis, aneurysm, arteriovenous malformation, and fistulas are a few other neurovascular disorders that can be treated with the help of neurovascular devices.
Central nervous system or brain vascular disorders are diagnosed and treated with interventional neurology technologies. Interventional neurology includes angiography, endovascular operations, fluoroscopy,and catheter-based procedures. For example, several neurological illnesses, such as arteriovenous malformations,cerebral aneurysm,arteriovenous fistula, intracranial stenosis, and vasculitis, can be diagnosed with catheter angiography, one of the earliest in-vivo brain vascular imaging procedures.
Due to the decreased danger and trauma involved with these treatments, minimally invasive procedures are becoming increasingly common. In addition, smaller incisions reduce postoperative pain and speed up healing, resulting in high adoption of these operations and increasing R&D in this area. To bring cutting-edge minimally invasive surgical equipment to market, several important firms are investing in R&D. One of the minimally invasive procedures that doctors highly suggest is the endovascular coiling used to treat cerebral aneurysms.
During this surgery, a microcatheter is placed via the aneurysm-containing artery in the groin area. Platinum coils are released to encourage aneurysm coagulation and stop blood from entering other parts of the brain. The considerable volume of neurovascular devices consumed, which has contributed to the market expansion, is primarily due to the fast-expanding patient population for the target disorders across the key markets.
COVID-19 Impact Analysis
The neurovascular market has suffered due to the limited accessibility of medical care for disorders other than COVID-19 globally. Another element that has a detrimental impact on the market for neurovascular devices is the scarce availability of medical personnel globally. The market has also been negatively influenced by surgeries that aren't essential and can be delayed, such as those for brain aneurysms. On the other hand, the ischemic stroke section is less affected since people who have an ischemic stroke need immediate medical intervention.Market Growth Factors
Growing demand for minimally invasive procedures
Minimally invasive medical techniques offer more therapeutic benefits than traditional surgical procedures, including a shorter hospital stay, quicker patient recovery, increased procedure effectiveness and safety, and lower costs. Hence, market acceptability and demand for minimally invasive surgical treatments are increasing among medical professionals globally. Elderly patients are more prone to neurological and neurovascular disorders. They choose treatment methods that offer simple diagnosis, straightforward treatments, increased safety, the shortest hospital stay feasible, superior efficacy, and affordable costs. These factors are estimated to support the expansion of the neurovascular devices market in the near future.Growing prevalence of strokes over the globe
Strokes are among the leading cause of mortality worldwide, and the prevalence is very high in the United States. Globally, there are more strokes than ever before because of an array of reasons, including growing urbanization, a sedentary lifestyle, and stress. Also, the condition significantly contributes to the annual mortality toll globally. These trends are creating a sizable patient base that has to be treated. Throughout the forecast period, it is predicted that this, along with rising patient awareness regarding the availability of treatment and reimbursement for neurovascular disorders, particularly in emerging nations, will further accelerate the expansion of the neurovascular devices market.Market Restraining Factor
High cost to be spent on neurovascular surgeries & relative products
The high price of neurovascular procedures and equipment is a major issue that is constraining the market for neurovascular devices, particularly in emerging nations having poor reimbursement policies. In addition, the cost of maintenance and any other associated indirect charges contribute to an increase in the overall price of these devices, which in turn limits the adoption of these technologies. Due to financial constraints, freestanding ambulatory surgery centers and small hospitals are less likely to invest in costly and advanced technologies. Thus, the high cost of neurovascular procedures may decline the growth of the neurovascular devices market in the upcoming years.Technology Outlook
Based on technology, the neurovascular devices market is segmented into embolization & coiling, cerebral balloon angioplasty & stenting systems, support devices, and neuro-thrombectomy devices. In 2021, the embolization & coiling devices segment held the highest revenue share in the neurovascular devices market. Neurovascular conditions such as carotid artery diseases, arteriovenous malformations (AVMs), and intracranial atherosclerotic diseases frequently necessitate embolization devices. In addition, other factors, including the rapid introduction of new and innovative neurovascular devices, contribute to the market's expansion.Disease Pathology Outlook
On the basis of disease pathology, the neurovascular devices market is classified into ischemic strokes, cerebral aneurysm, carotid artery stenosis, arteriovenous malformations & fistulas and other diseases. The ischemic stroke segment acquired a substantial revenue share in the neurovascular devices market in 2021. Globally, ischemic stroke is becoming more common. To avoid ischemic stroke in patients, the American Heart Association advises using stent retrieval devices to remove blood clots. The need for neurovascular devices is expected to rise in the coming years due to the rising number of cases of ischemia.End-user Outlook
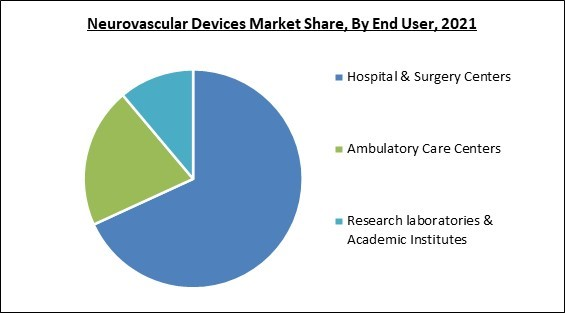
By end user, the neurovascular devices market is bifurcated into hospitals & surgical centers, ambulatory care centers, and research laboratories & academic institutes. The ambulatory care centers segment recorded a remarkable revenue share in the neurovascular devices market in 2021. Patients' decreased wait times at ASCs are anticipated to drive the segment's significant growth during the projected period. In addition, the segment's growth is propelling due to the presence of skilled professionals in such healthcare facilities.Regional Outlook
Region wise, the neurovascular devices market is analyzed across North America, Europe, Asia Pacific and LAMEA. In 2021, the North America region dominated the neurovascular devices market with the largest revenue share. The neurovascular devices market in North America is mainly fueled by variables such as the substantial disease burden, the increase in minimally invasive neurology treatments, and the establishment of trauma/emergency care facilities in the United States. In addition, technological advances and increased research and development activities resulting in product releases are driving regional market expansion.The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Abbott Laboratories, Medtronic PLC, Stryker Corporation, Johnson & Johnson, Terumo Corporation, Merit Medical Systems, Inc., MicroPort Scientific Corporation, Medikit Co. Ltd., and W. L. Gore & Associates, Inc.
Scope of the Study
By End-user
- Hospital & Surgery Centers
- Ambulatory Care Centers
- Research laboratories & Academic Institutes
By Technology
- Embolization & Coiling
- Embolic Coils
- Flow Diversion Devices
- Liquid Embolic Agents
- Neurothrombectomy Devices
- Clot Retrievers
- Suction & Aspiration Devices
- Snares
- Cerebral Balloon Angioplasty & Stenting Systems
- Carotid Artery Stents
- Embolic Protection Systems
- Support Devices
- Microcatheters
- Microguidewires
By Disease Pathology
- Cerebral Aneuryms
- Ischemic Strokes
- Cartoid Artery Stenosis
- Arteriovenous Malformation & Fistulas (AVM)
- Others
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Abbott Laboratories
- Medtronic PLC
- Stryker Corporation
- Johnson & Johnson
- Terumo Corporation
- Merit Medical Systems, Inc.
- MicroPort Scientific Corporation
- Medikit Co. Ltd.
- W. L. Gore & Associates, Inc.
Unique Offerings
- Exhaustive coverage
- The highest number of market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Chapter 1. Market Scope & Methodology
Chapter 2. Market Overview
Chapter 3. Global Neurovascular Devices Market by End-user
Chapter 4. Global Neurovascular Devices Market by Technology
Chapter 5. Global Neurovascular Devices Market by Disease Pathology
Chapter 6. Global Neurovascular Devices Market by Region
Chapter 7. Company Profiles
Companies Mentioned
- Abbott Laboratories
- Medtronic PLC
- Stryker Corporation
- Johnson & Johnson
- Terumo Corporation
- Merit Medical Systems, Inc.
- MicroPort Scientific Corporation
- Medikit Co. Ltd.
- W. L. Gore & Associates, Inc.
Methodology

LOADING...