Speak directly to the analyst to clarify any post sales queries you may have.
Framing the modern analgesics environment through clinical, regulatory, and commercial lenses to guide strategic portfolio and policy decisions
The analgesics landscape stands at a crossroads shaped by evolving clinical practice, heightened regulatory scrutiny, and shifting patient expectations. Recent advances in multimodal pain management, combined with a persistent need to balance efficacy and safety, have placed analgesics at the center of both acute care optimization and chronic disease management. Meanwhile, payer strategies and hospital protocols continue to influence formulary selections and prescribing patterns, pushing manufacturers and suppliers to refine value propositions that emphasize outcomes, safety profiles, and supply reliability.In this context, manufacturers of both branded and generic analgesics must navigate a complex ecosystem that includes diverse routes of administration, heterogeneous patient populations, and region-specific regulatory frameworks. Clinical evidence is increasingly moving toward targeted, indication-specific approaches that aim to reduce opioid exposure for acute pain while maintaining access for complex chronic pain conditions. As a result, innovation is occurring not only in molecular development but also in delivery systems, combination therapies, and care-model integration.
Transitioning from broad categorization to actionable insight requires a disciplined approach to data synthesis, rigorous evaluation of clinical and commercial evidence, and a clear understanding of distribution dynamics across inpatient and outpatient settings. This executive summary frames the critical forces reshaping the analgesics sector and sets the stage for pragmatic recommendations that help stakeholders prioritize investments and adapt to emerging care paradigms.
Charting the pivotal clinical, regulatory, and commercial inflection points that are reshaping treatment paradigms and supplier strategies across the analgesics sector
Over the past several years, transformative shifts have redefined how analgesics are developed, prescribed, and delivered. Clinically, there has been a measurable move toward multimodal pain management that reduces reliance on single-agent opioid therapy. This approach combines pharmacologic and non-pharmacologic interventions, encouraging the adoption of non-opioid alternatives and adjunctive modalities that improve recovery trajectories and reduce adverse events. Concurrently, regulators and payers have intensified focus on prescribing safeguards, post-marketing surveillance, and risk mitigation strategies that place a premium on therapies with demonstrable safety benefits.On the commercial front, the balance of power continues to shift toward value-based contracting and outcomes-linked arrangements. Manufacturers are responding by generating real-world evidence and designing patient-centered support programs. Innovations in delivery, such as extended-release formulations and transdermal systems, are gaining traction because they promise improved adherence and more consistent pharmacokinetic profiles. At the same time, the generic sector is evolving through consolidation and competitive differentiation based on supply chain reliability and manufacturing quality standards.
Technological change is also influencing care pathways; telehealth and digital therapeutics are increasingly integrated into pain management strategies, enabling remote monitoring and adherence interventions that support safer prescribing. Taken together, these shifts emphasize a marketplace that rewards clinical differentiation, supply resilience, and evidence of real-world benefit, creating both opportunities and imperatives for incumbent and new entrants alike.
Examining how recent tariff alterations are driving supply chain reconfiguration, procurement strategy shifts, and operational resilience planning across analgesics manufacturing
Recent tariff policy changes in the United States have introduced a material set of considerations for companies operating within global analgesics supply chains. Tariff adjustments influence sourcing choices for active pharmaceutical ingredients and finished-dose formulations, prompting manufacturers to reassess supplier diversification, contract terms, and nearshoring opportunities. Given the concentration of certain raw material production in specific geographies, increased import duties translate into a need for proactive procurement strategies and renegotiated supplier agreements to preserve margin and uninterrupted supply to clinical channel partners.In response, firms are intensifying efforts to map upstream inputs and qualify alternative suppliers that meet regulatory and quality standards. This includes evaluating domestic manufacturing capacity, leveraging toll-manufacturing relationships, and increasing inventory buffers for critical components. From a commercial perspective, payers and health systems are attentive to cost dynamics and may adjust procurement preferences based on total landed cost and demonstrated supply stability. Consequently, companies must provide transparent supply chain narratives and contingency planning to reassure buyers and maintain formulary positioning.
Regulatory compliance and customs-related documentation have also become more prominent features of supplier audits; therefore, organizations should invest in enhanced trade-compliance capabilities and scenario planning. Through deliberate reshoring initiatives or strategic partnerships, stakeholders can mitigate tariff-driven volatility while aligning operational resilience with evolving policy landscapes and clinical demand signals.
Using multidimensional segmentation across drug class, delivery route, clinical indication, and distribution channel to align product strategy with real-world care pathways
Granular segmentation provides a structural lens for interpreting clinical demand patterns and commercial opportunities across the analgesics continuum. When viewed by type of drug, the market divides into non-opioids and opioids, where non-opioids include acetaminophen, non-steroidal anti-inflammatory drugs, and salicylates, and opioids include codeine, fentanyl, hydrocodone, morphine, and oxycodone. Each category presents distinct efficacy, safety, and regulatory profiles that inform product positioning and lifecycle strategy. For example, non-opioid agents are increasingly preferred for mild-to-moderate acute pain and as components of multimodal regimens, whereas the opioid class remains indispensable for severe acute pain and certain chronic conditions, albeit with intensified stewardship requirements.Considering route of administration, stakeholders should evaluate intramuscular and intravenous options for inpatient acute care, oral formulations such as capsules and tablets for ambulatory management, and rectal, topical, and transdermal systems to address specific clinical needs and adherence challenges. Delivery format directly influences dosing convenience, onset of action, and suitability across care settings, which in turn shapes procurement and distribution decisions. Indication-based segmentation further refines commercial focus: acute pain-both injury-related and postoperative-drives short-course, high-intensity demand in perioperative and emergency contexts, while chronic pain categories, including arthritis, back pain, cancer pain, and neuropathic pain, generate sustained, long-term utilization that emphasizes tolerability and risk management.
Distribution channel analysis spans hospital pharmacies, online pharmacies, and retail pharmacies, each of which has unique procurement cycles, margin structures, and regulatory touchpoints. Hospital pharmacies prioritize parenteral formulations and institutional contracting efficiencies, while retail and online channels emphasize convenience, adherence support, and patient education. By integrating type, administration route, indication, and channel, organizations can better align product design, evidence generation, and commercial tactics to distinct buyer requirements and patient journeys.
Evaluating geographically driven clinical practices, regulatory regimes, and commercial models to inform differentiated regional go-to-market approaches
Regional dynamics exert a strong influence on clinical practice, regulatory expectations, and commercial models in the analgesics space. In the Americas, prescriber guidelines and payer mechanisms increasingly emphasize stewardship and outcomes measurement, driving demand for products that demonstrate safety advantages and measurable benefits in recovery metrics. Suppliers serving this region should prioritize engagement with integrated health systems, invest in real-world evidence generation, and develop patient support programs that address adherence and misuse mitigation.Across Europe, Middle East & Africa, diverse regulatory frameworks and heterogenous access environments require flexible market-entry strategies and adaptive pricing approaches. Western European markets commonly demand robust clinical comparators and health-technology assessment dossiers, whereas emerging markets in the broader region may prioritize cost-effective generics and scalable distribution models. Consequently, companies must calibrate clinical development and commercial deployment to accommodate both high-evidence procurement in mature markets and cost-sensitive distribution in developing markets.
The Asia-Pacific region presents a mix of rapid urbanization, expanding hospital capacity, and growing outpatient care networks. Local manufacturing capabilities and regional supply hubs are increasingly important, and partnerships with regional distributors can accelerate market penetration. In addition, demographic trends such as aging populations in specific Asia-Pacific markets are heightening prevalence of chronic pain conditions, which in turn supports demand for sustained-delivery technologies and long-term therapeutic support programs. Strategic regional planning must therefore balance regulatory navigation, local partnerships, and targeted evidence generation to maximize access and uptake.
Analyzing the competitive landscape where R&D-driven differentiation, generic scale, and strategic alliances determine access, pricing, and formulary placement
Competitive dynamics in the analgesics sector are shaped by a mix of multinational pharmaceutical companies, specialty manufacturers, and generic producers, each pursuing differentiated strategies to capture clinical preference and payer acceptance. Established multinational firms leverage broad R&D capabilities and global regulatory experience to advance novel formulations and indication expansions, prioritizing clinical differentiation and branded value propositions. Specialty manufacturers and smaller innovators often focus on delivery technologies, niche indications, or companion support services that enable targeted market entry without requiring large-scale clinical programs.The generic segment remains a powerful force, emphasizing cost competitiveness, manufacturing scale, and supply reliability. Firms that demonstrate robust quality systems, capacity redundancy, and rapid regulatory filings are often advantaged in tender-based procurement environments. At the same time, new commercial models-such as outcomes-linked agreements and bundled purchasing-are creating openings for organizations that can provide verifiable real-world performance metrics and integrated patient support.
Partnerships, licensing, and strategic alliances are increasingly common paths to scale and to accelerate access to complementary technologies. Companies that invest in digital adherence tools, clinician education programs, and integrated value demonstrations tend to secure stronger formulary positions. Given these dynamics, competitive success requires a balanced focus on clinical evidence, manufacturing excellence, and commercial flexibility, underpinned by a clear articulation of product differentiation and supply-chain assurances.
Implementing an integrated strategy that harmonizes clinical differentiation, supply-chain resilience, and payer-facing value propositions to capture durable market advantage
Leaders seeking to strengthen their position in the analgesics market should adopt a portfolio approach that addresses clinical differentiation, supply resilience, and payer alignment. First, focus R&D and lifecycle investments on formulations and delivery systems that reduce adverse events and improve adherence, supported by robust real-world evidence programs that resonate with payers and health systems. Second, fortify supply-chain strategies by qualifying multiple suppliers, investing in domestic or regional manufacturing capacity where feasible, and implementing transparent contingency planning that can be communicated to customers and partners.Moreover, commercial teams should prioritize value-based conversations with payers and integrated delivery networks, offering pilot programs and outcomes-tracking frameworks that demonstrate tangible benefits. In parallel, deploy targeted education and stewardship initiatives that aid clinicians in adopting multimodal regimens and safe prescribing practices. From a go-to-market perspective, tailor channel strategies to the distinct needs of hospital pharmacies, online pharmacies, and retail pharmacies, ensuring that commercial models address purchasing cycles, reimbursement dynamics, and patient support requirements.
Finally, consider strategic collaborations to accelerate market entry and expand service offerings. Whether through licensing agreements, co-marketing partnerships, or technology integrations, alliances can provide rapid access to distribution networks, digital tools, and complementary clinical capabilities that enhance overall value propositions.
Describing a rigorous mixed-methods research framework that combines primary expert insights with systematic secondary analysis and scenario validation
This report’s findings are grounded in a mixed-methods research approach that integrates primary conversations with clinicians, procurement leaders, and industry experts alongside comprehensive secondary analysis of regulatory documents, clinical literature, and trade data. Primary research included structured interviews and expert panels designed to surface practitioner behavior, formulary decision criteria, and real-world operational constraints. Secondary sourcing involved systematic review of peer-reviewed journals, clinical guidelines, regulatory communications, and publicly available manufacturing and trade records to triangulate trends and validate hypotheses.Analytical methods encompassed qualitative thematic synthesis to interpret clinician and payer attitudes, and quantitative descriptive analysis to map supply chain flows and distribution channel characteristics. Scenario analysis and sensitivity testing were employed to examine the implications of policy shifts and supply disruptions. Throughout the process, data quality controls, source triangulation, and expert validation were applied to ensure credibility and reduce bias. Where possible, findings were checked against multiple independent sources and refined via iterative consultations with domain specialists to ensure relevance and practical utility for decision-makers.
Summarizing strategic imperatives that combine clinical evidence, operational resilience, and regionally tailored commercial execution to secure long-term value
In conclusion, the analgesics sector is undergoing a period of substantive change driven by clinical best-practice evolution, regulatory emphasis on safety and stewardship, and commercial pressures that favor evidence-based value propositions and supply reliability. Stakeholders who proactively invest in differentiated formulations, robust real-world evidence, and resilient manufacturing and procurement strategies will be best positioned to navigate tariff volatility and shifting payer expectations. At the same time, alignment with evolving care models-such as multimodal pain management and digitally enabled patient support-will be essential to capture long-term clinical and commercial opportunities.Moving forward, organizations should prioritize adaptive planning that integrates clinical, regulatory, and operational foresight, enabling rapid response to policy changes and emerging clinical data. By coupling disciplined evidence generation with pragmatic supply-chain measures and targeted regional strategies, companies can both mitigate near-term disruptions and create sustainable differentiation in a complex and highly scrutinized market. The insights summarized here aim to equip leaders with the perspective necessary to make informed strategic choices and to operationalize initiatives that deliver measurable benefit across care settings.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Analgesics Market
Companies Mentioned
The key companies profiled in this Analgesics market report include:- Abbott Laboratories
- Bayer AG
- Bristol-Myers Squibb Company
- C.H. Boehringer Sohn AG & Co. KG
- Cipla Limited
- CSPC Pharmaceutical Group
- Dr. Reddy’s Laboratories Ltd.
- Eli Lilly and Company
- GlaxoSmithKline plc
- Grünenthal GmbH
- Haleon plc
- Hikma Pharmaceuticals plc
- Johnson & Johnson Services, Inc.
- Lupin Limited
- Mallinckrodt plc
- Merck KGaA
- Novartis AG
- Perrigo Company plc
- Pfizer Inc.
- Reckitt Benckiser Group plc
- Sanofi S.A.
- Sun Pharmaceutical Industries Limited
- Teva Pharmaceutical Industries Ltd.
- Viatris Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 187 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
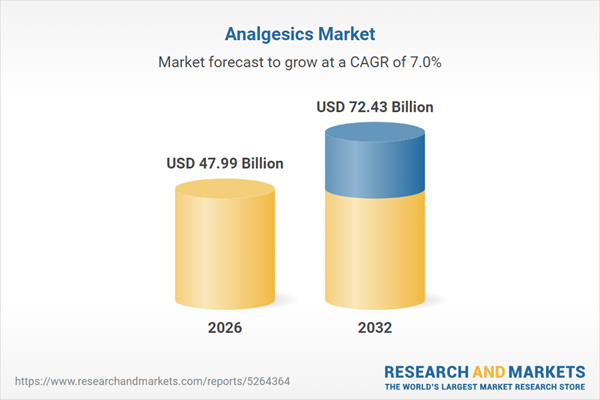
| Estimated Market Value ( USD | $ 47.99 Billion |
| Forecasted Market Value ( USD | $ 72.43 Billion |
| Compound Annual Growth Rate | 7.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |