Speak directly to the analyst to clarify any post sales queries you may have.
A concise orientation to the contemporary angiographic catheter environment highlighting clinical drivers, device evolution, and procurement dynamics
The field of angiographic catheters is at an inflection point driven by converging clinical imperatives and technological refinement. Advances in material science, device coatings, and microfabrication techniques have translated into catheters that navigate complex vascular anatomies with improved trackability and reduced vessel trauma. At the same time, procedural trends in interventional cardiology, neurointervention, and peripheral vascular therapy are reshaping device requirements, emphasizing smaller profiles, enhanced torque response, and compatibility with adjunctive imaging and therapeutic tools.
Regulatory scrutiny and heightened attention to device safety have increased the emphasis on biocompatibility and manufacturing controls, prompting device makers to prioritize validated processes and robust post-market surveillance. Meanwhile, hospital systems and ambulatory care providers are balancing clinical performance against procedural efficiency and cost-of-care objectives, prompting procurement teams to consider total procedural value rather than unit price alone. As stakeholders adapt, opportunities arise for device developers who can demonstrate clear clinical advantages, reproducible performance, and supply chain resilience.
Through this report, readers will gain a synthesized view of how clinical drivers, material choices, procedural settings, and regulatory dynamics intersect to influence product design priorities, purchasing behavior, and strategic investment decisions across the angiographic catheter ecosystem.
How converging clinical expectations, materials innovation, and shifting care settings are redefining product priorities and supply strategies across the perfusion device ecosystem
The landscape for angiographic catheters is being reshaped by transformative shifts that extend beyond incremental device improvements to strategic changes in care delivery and supply ecosystems. The first pivot reflects a transition in device expectations: clinicians increasingly demand catheters that support multimodal procedures, combining imaging, diagnostic access, and therapeutic delivery without instrument exchanges. This trend incentivizes designs that balance lumen architecture, shaft flexibility, and tip control while maintaining compatibility with adjunctive guidewires and imaging catheters.
Concurrently, materials and coating technologies have migrated from single-purpose solutions to integrated packages that enhance lubricity, reduce thrombogenicity, and extend usable lifespan when reprocessing is applicable. These technical advances occur in parallel with changing procedural venues; ambulatory surgical centers and outpatient interventional suites are performing a broader array of catheter-based therapies, driving demand for single-use systems that streamline turnover and reduce infection risk. Moreover, digital health integration-such as intra-procedural analytics and device traceability-adds a new layer of value, enabling quality assurance and post-market data collection.
Finally, strategic sourcing and manufacturing strategies are evolving as medical device companies seek flexibility against geopolitical pressures and material shortages. Firms that can diversify supply chains, adopt modular manufacturing, and demonstrate traceable sourcing for critical polymers and coatings will be better positioned to respond to procurement and regulatory expectations worldwide.
The 2025 tariff-induced adjustments that reshaped sourcing choices, manufacturing localization, and procurement priorities for vascular access device manufacturers
Policy measures implemented in the United States in 2025 introduced tariff pressures that reverberated across medical device supply chains, and angiographic catheters were no exception. Tariff-related cost increases on imported raw materials and certain finished components compelled manufacturers to reassess sourcing strategies and contractual terms with suppliers. In response, several firms accelerated supplier diversification, sought alternative polymers and coating vendors, and reexamined inventory management to protect production continuity and preserve delivery timelines to hospitals and clinics.
These dynamics prompted intensified conversations among procurement, regulatory, and quality teams about cost-to-performance trade-offs. Some organizations adopted localized sourcing and increased domestic manufacturing capacity to reduce exposure to import tariffs, which in turn influenced capital allocation for manufacturing equipment and validated cleanroom space. Others renegotiated agreements with contract manufacturers to share the burden of duty-related cost increases through longer-term commitments or adjusted pricing frameworks.
At the clinical interface, purchasing stakeholders weighed the impact of tariff-influenced cost pressures against the imperative of maintaining device availability and safety. Consequently, procurement decisions incorporated a broader set of criteria-resilience of supply, transparency of the supplier network, and the ability to provide validated substitution options for critical components-so that procedural performance and patient safety remained paramount despite macroeconomic headwinds.
Comprehensive segmentation synthesis connecting product subtypes, clinical use cases, material choices, and care settings to inform R&D and procurement priorities
Deconstructing the angiographic catheter universe along product, application, end-user, material, and usage dimensions reveals differentiated technical and commercial imperatives that inform strategy. Product segmentation spans Balloon, Diagnostic, Guiding, and Specialty catheters, and each subtype carries subcategories with distinct design constraints and clinical roles. Balloon catheters, evaluated across Monorail and OvertheWire platforms, must balance rapid exchange convenience with profile control for coronary and peripheral therapies. Diagnostic catheters, available in Curved and Straight geometries, are optimized for selective coronary and cerebral access and must deliver consistent torque response and radiopacity. Guiding catheters, distinguished by Hydrophilic Coated and Non-Coated finishes, prioritize support and compatibility with interventional toolsets, while Specialty devices such as Flow Diverters and Microcatheters are purpose-built for complex neurovascular interventions and require ultra-fine tolerances and advanced polymer formulations.
Application-focused distinctions underscore differing performance demands between Coronary, Neurovascular, and Peripheral procedures. Coronary applications emphasize minute profile control and rapid lesion access; neurovascular work demands extreme navigability and atraumatic tips; peripheral procedures require durability for longer vascular paths and often larger lumens. End users present heterogenous procurement environments: Ambulatory Surgical Centers prioritize streamlined supply chains and disposability to maintain throughput, Cardiac Catheterization Laboratories demand high-performance and device interoperability for complex interventions, Clinics seek cost-effective diagnostic tools, and Hospitals balance acute-care readiness with inventory breadth.
Material choices among Polymer, Silicone, and Teflon influence flexibility, lubricity, and biocompatibility, shaping device interaction with blood flow and vessel walls. Finally, usage paradigms-Reusable versus Single Use-affect lifecycle considerations, sterilization validation needs, and economic assessments at the point of care, thereby shaping R&D investments and commercialization strategies.
Regional dynamics across the Americas, Europe Middle East & Africa, and Asia-Pacific that determine adoption curves, procurement behaviors, and go-to-market strategies
Regional dynamics introduce distinct regulatory, reimbursement, and infrastructure variables that shape clinical adoption and commercial strategy. In the Americas, established procedural volumes and advanced interventional programs in cardiology and peripheral care support demand for a breadth of catheter solutions, while procurement cycles and hospital-contracted buying consortia influence vendor selection and product standardization. Advanced imaging integration and a mature supply-chain infrastructure encourage adoption of premium-feature devices that demonstrate procedural efficiency and measurable clinical outcomes.
The Europe, Middle East & Africa geographies present a mosaic of regulatory environments and care delivery models, where single-payer systems in parts of Europe emphasize cost-effectiveness and real-world evidence for adoption, while emerging markets in the Middle East and Africa prioritize access and supply resilience. In these regions, modular product portfolios that can be tailored to local clinical practices and inventory constraints enjoy better market traction, and partnerships with local distributors are often pivotal for regulatory navigation and clinician training.
Asia-Pacific exhibits rapid procedural growth in both coronary and neurovascular interventions, accompanied by significant investment in capacity building and domestic manufacturing. Diverse payer structures and variable hospital capabilities create opportunities for differentiated strategies that combine high-performance offerings in tertiary centers with cost-conscious models for broader adoption. Across all regions, harmonizing regulatory dossiers and demonstrating supply-chain transparency remain critical to facilitating cross-border commercialization and clinician confidence.
Competitive landscape analysis revealing how scale, specialization, partnerships, and manufacturing capabilities shape strategic differentiation and clinician adoption
Competitive dynamics among device manufacturers are characterized by a mix of scale-driven incumbents and specialized innovators who together shape product evolution. Larger diversified medical technology firms leverage integrated R&D, global manufacturing footprints, and established distributor networks to deliver comprehensive portfolios spanning guiding, diagnostic, and balloon catheters. Their capabilities in regulatory filing, clinical trial sponsorship, and post-market surveillance enable them to support hospital systems and integrated delivery networks that prioritize proven performance and vendor stability.
At the same time, smaller specialist companies and agile start-ups drive material innovation, microfabrication techniques, and niche device concepts-particularly in neurovascular microcatheter and flow diverter design-where highly focused expertise accelerates iteration cycles. Contract manufacturers and component suppliers play a pivotal role by offering validated polymer processing, coating applications, and cleanroom assembly that allow brand owners to scale without duplicating heavy capital investments.
Partnerships and co-development agreements have become common mechanisms for bridging gaps between clinical need and manufacturable solutions. Collaborations with academic centers and clinician thought leaders facilitate rapid prototyping and usability testing, while alliances with distributor partners expand reach into ambulatory and international markets. Strategic differentiation increasingly hinges on the ability to combine demonstrable clinical outcomes with reliable supply continuity and comprehensive training and support services for clinicians.
Actionable strategic imperatives for device manufacturers that link interoperable product design, supply diversification, clinical support, and digital traceability to sustained adoption
Industry leaders must adopt a multi-pronged strategy that aligns product innovation with operational resilience and clinician-centered value propositions. First, companies should prioritize design-for-interoperability that reduces procedural friction by ensuring catheter compatibility with a wide array of guidewires, imaging tools, and adjunct devices; this will enhance clinician preference and simplify hospital purchasing decisions. Second, investing in advanced materials and coating technologies that demonstrably improve lubricity, reduce thrombotic risk, and extend functional reliability will create clear clinical differentiation and support premium positioning.
Operationally, diversifying the supplier base for critical polymers and components and creating validated dual-sourcing strategies will mitigate the impact of tariff shifts and geopolitical disruptions. Parallel investments in regional manufacturing capabilities or strategic partnerships with contract manufacturers can reduce lead times and improve responsiveness to demand fluctuations. Commercially, companies should develop service bundles that include clinician training, procedural support, and data-driven post-market evidence to strengthen product adoption and justify value-based procurement.
Finally, embedding traceability and digital tools to capture procedural performance and device analytics will enable continuous improvement and create defensible evidence for outcomes-based contracting. By integrating these measures, manufacturers can enhance clinical trust, operational agility, and long-term commercial resilience.
Transparent methodological overview combining clinician interviews, regulatory review, and analytical triangulation to ensure reproducible and balanced device insights
This analysis synthesizes primary and secondary research inputs to ensure a balanced and reproducible perspective on the angiographic catheter ecosystem. Primary research involved structured interviews with clinicians across coronary, neurovascular, and peripheral specialties, procurement leaders from hospitals and ambulatory surgical centers, and executives from device manufacturers and contract production partners. These stakeholder conversations were designed to capture device performance priorities, procurement criteria, and first-hand observations of supply-chain disruptions and material constraints.
Secondary research encompassed peer-reviewed clinical literature, regulatory guidance documents, and publicly available technical resources on polymer and coating technologies relevant to catheter construction. Data triangulation was employed to validate thematic findings and to reconcile differing perspectives across geographies and care settings. Where applicable, clinical practice guidelines and consensus statements were reviewed to align device performance attributes with procedural requirements.
Analytical methods included qualitative thematic coding of interview transcripts, comparative assessment of product feature sets across device subtypes, and scenario analysis to evaluate the implications of supply-chain and policy shifts. Throughout the research process, emphasis was placed on transparency of source attribution, reproducibility of thematic coding, and the inclusion of divergent stakeholder viewpoints to avoid single-source bias.
A forward-looking synthesis explaining how innovation, operational resilience, and clinician-focused evidence will determine competitive success and clinical impact
The angiographic catheter sector is poised to advance through integrated technical, operational, and commercial initiatives that align with evolving clinical needs. Innovations in materials, coatings, and microfabrication are enabling devices that better navigate complex anatomies and support multimodal procedures, while changing care settings and procurement priorities are creating demand for both high-performance and cost-effective solutions. In this context, manufacturers and health systems must adopt strategies that reconcile clinical performance with supply resilience and procedural efficiency.
Looking ahead, differentiation will increasingly depend on the ability to provide validated clinical benefits, seamless interoperability with existing procedural kits and imaging systems, and demonstrable supply-chain transparency. Additionally, the expansion of ambulatory procedural venues and the maturation of neurovascular interventions create targeted opportunities for both specialty device designers and broader portfolio manufacturers. By focusing on clinician-centered design, validated materials, and strategic manufacturing choices, stakeholders can navigate policy pressures and shifting procurement expectations while maintaining a trajectory toward safer, more efficient patient care.
In conclusion, a pragmatic combination of innovation, operational redundancy, and evidence generation will position organizations to meet clinician needs and to capitalize on procedural advances across coronary, neurovascular, and peripheral domains.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Angiographic Catheters Market
Companies Mentioned
The key companies profiled in this Angiographic Catheters market report include:- Abbott Laboratories
- AngioDynamics Inc
- Argon Medical Devices Inc
- Asahi Intecc Co Ltd
- B Braun Melsungen AG
- Becton Dickinson and Company
- Biotronik SE & Co KG
- Boston Scientific Corporation
- Cardinal Health Inc
- Cook Medical LLC
- Cordis Corporation
- Lepu Medical Technology Co Ltd
- Medtronic plc
- Merit Medical Systems Inc
- MicroPort Scientific Corporation
- Nipro Corporation
- Penumbra Inc
- Teleflex Incorporated
- Terumo Corporation
- Vascular Solutions Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
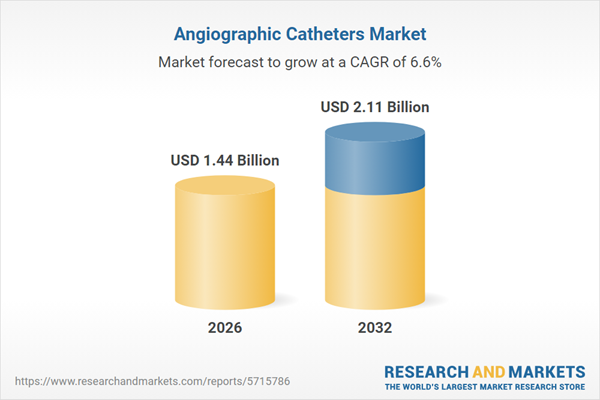
| Estimated Market Value ( USD | $ 1.44 Billion |
| Forecasted Market Value ( USD | $ 2.11 Billion |
| Compound Annual Growth Rate | 6.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |