Antibacterial Drugs - Key Trends and Drivers
Antibacterial drugs, commonly known as antibiotics, are essential in the treatment of bacterial infections and are a cornerstone of modern medicine. These drugs work by either killing bacteria (bactericidal) or inhibiting their growth (bacteriostatic), depending on their mechanism of action. Antibacterial drugs are classified into several categories, including penicillins, cephalosporins, tetracyclines, macrolides, fluoroquinolones, and aminoglycosides, each targeting specific types of bacteria. For example, penicillins and cephalosporins disrupt bacterial cell wall synthesis, while tetracyclines inhibit protein synthesis. These drugs are used to treat a wide range of bacterial infections, from minor skin infections and respiratory tract infections to more severe conditions such as sepsis, bacterial meningitis, and tuberculosis. The discovery and widespread use of antibiotics have dramatically reduced the morbidity and mortality associated with bacterial infections, representing one of the most significant advancements in medical history.A significant trend in the antibacterial drugs market is the increasing problem of antibiotic resistance, where bacteria evolve mechanisms to withstand the effects of antibiotics. This phenomenon has led to the rise of 'superbugs' which are resistant to multiple antibiotics and pose a severe threat to public health. The World Health Organization (WHO) has identified antibiotic resistance as one of the top global health threats. In response to this crisis, there is a growing emphasis on developing new antibiotics and alternative therapies. Research is being directed towards bacteriophages, viruses that specifically infect and kill bacteria, and antimicrobial peptides, which can disrupt bacterial membranes. Efforts are also being made to promote the responsible use of antibiotics through stewardship programs aimed at reducing overuse and misuse, which are primary drivers of resistance. These programs focus on educating healthcare professionals and the public about the importance of appropriate antibiotic use and the potential consequences of resistance. Pharmaceutical companies and research institutions are investing heavily in innovative research to discover novel antibacterial agents and repurpose existing drugs to combat resistant strains, ensuring that effective treatments remain available.
The growth in the antibacterial drugs market is driven by several factors, including technological advancements in drug discovery, the increasing prevalence of bacterial infections, and the rising awareness of antibiotic resistance. Technological advancements, such as high-throughput screening, genomics, and bioinformatics, have accelerated the discovery of new antibiotics and enhanced the understanding of bacterial resistance mechanisms. These technologies enable researchers to identify novel drug targets and develop more effective antibacterial agents. The growing prevalence of bacterial infections, exacerbated by factors such as aging populations, increased hospitalizations, and the rising incidence of chronic diseases, continues to drive demand for effective antibacterial treatments. Additionally, public health initiatives and educational campaigns have raised awareness about the dangers of antibiotic resistance, leading to increased demand for new and more effective antibacterial drugs. The development of rapid diagnostic tools to identify bacterial infections and resistance patterns is also propelling market growth, as these tools enable more targeted and effective treatments. Furthermore, the support from government and non-government organizations in funding research and development activities, as well as regulatory incentives such as expedited review processes for new antibiotics, is fostering innovation in the market. Overall, the combination of these factors is driving sustained growth and innovation in the antibacterial drugs market, ensuring the continued effectiveness of these critical medicines in the fight against bacterial infections.
Report Scope
The report analyzes the Antibacterial Drugs market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Drug Class (ß-lactams, Quinolones, Macrolides, Tetracyclines, Aminoglycosides, Other Drug Classes); Distribution Channel (Hospital Pharmacies, Drug Stores & Retail Pharmacies, Online Sales).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the ß-lactams segment, which is expected to reach US$35.4 Billion by 2030 with a CAGR of a 4.1%. The Macrolides segment is also set to grow at 4.3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $12.6 Billion in 2024, and China, forecasted to grow at an impressive 6.5% CAGR to reach $12.1 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Antibacterial Drugs Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Antibacterial Drugs Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Antibacterial Drugs Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Anika Therapeutics, Inc., Baxter International Inc., Ethicon Inc., FzioMed, Inc., Integra LifeSciences Holdings Corporation and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 124 companies featured in this Antibacterial Drugs market report include:
- Allergan PLC
- AstraZeneca PLC
- Bayer AG
- Bristol-Myers Squibb Company
- GlaxoSmithKline PLC
- Johnson & Johnson
- Merck & Co., Inc.
- Novartis International AG
- Pfizer, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Allergan PLC
- AstraZeneca PLC
- Bayer AG
- Bristol-Myers Squibb Company
- GlaxoSmithKline PLC
- Johnson & Johnson
- Merck & Co., Inc.
- Novartis International AG
- Pfizer, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 244 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
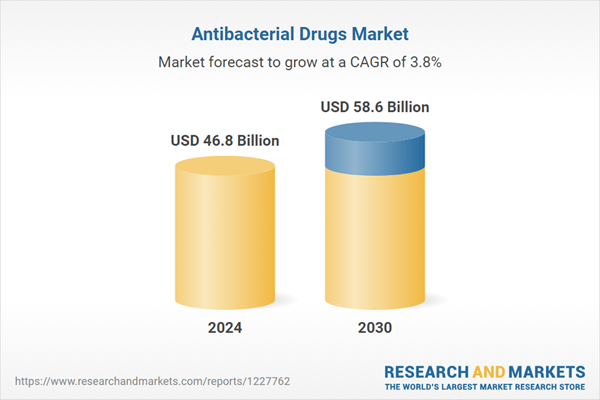
| Estimated Market Value ( USD | $ 46.8 Billion |
| Forecasted Market Value ( USD | $ 58.6 Billion |
| Compound Annual Growth Rate | 3.8% |
| Regions Covered | Global |