Speak directly to the analyst to clarify any post sales queries you may have.
A concise and strategic orientation to antibiotic resistance trends that frames clinical urgency alongside innovation pathways and operational imperatives for decision-makers
Antibiotic resistance represents one of the most complex and consequential public health challenges of the modern era, reshaping clinical practice, research priorities, and supply chain strategies across healthcare systems. The dynamics of resistance are driven by the interplay of microbial evolution, clinical prescribing behaviours, diagnostic capacity, and the pipeline of therapeutic innovations. Consequently, stakeholders must navigate an environment where traditional antimicrobial paradigms are under pressure and multifaceted responses are required to preserve therapeutic efficacy and patient outcomes.This executive summary synthesizes the strategic implications of current trends for healthcare providers, product developers, policy makers, and investors. It highlights how innovations in therapeutic modalities, shifting regulatory attitudes, and disruptions in global trade are changing opportunity spaces and risk profiles. By framing the issue through clinical impact, technology response, and market structure, the introduction establishes a foundation for the subsequent sections, enabling decision-makers to prioritize interventions that align clinical need with sustainable commercial models. The overall focus is on translating scientific and operational complexity into actionable insight that supports resilient planning and evidence-based investment.
How converging advances in therapeutics, diagnostics, regulation, and supply resilience are reshaping treatment pathways and commercial strategies in response to rising resistance
The landscape of antibiotic resistance is undergoing transformative shifts driven by both scientific innovation and systemic pressures that redefine how therapies are discovered, developed, regulated, and adopted. Advances in platform technologies are extending beyond small-molecule optimization to include biological approaches such as immunomodulators, monoclonal antibodies, and targeted phage therapies. These approaches are changing the value proposition for developers by offering pathogen-specific strategies that can complement or replace broad-spectrum agents, thereby altering clinical pathways and stewardship considerations.At the same time, diagnostics are improving the granularity and speed of pathogen identification, enabling more targeted therapy and reducing inappropriate antibiotic use. Regulatory frameworks are responding with adaptive pathways that accommodate novel modalities and incentivize development, while payers and providers increasingly demand evidence of real-world impact and cost-effectiveness. Meanwhile, supply chain resilience and manufacturing innovation are gaining strategic importance as stakeholders seek to ensure reliable access to critical therapeutics. Taken together, these shifts are accelerating a move toward integrated care models that marry precision diagnostics with tailored therapeutics, and they require coordinated investment in clinical evidence generation, reimbursement strategy, and distribution infrastructure.
Assessment of how recent tariff changes and trade policy dynamics are influencing supply chain resilience, manufacturing choices, and access dynamics for critical therapeutics
Recent trade policy changes have introduced renewed complexity into the procurement and distribution of antimicrobials and associated technologies, with tariff adjustments having a cascading effect on sourcing decisions, manufacturing footprints, and pricing strategies. Increased duties on imported active pharmaceutical ingredients, finished dosages, and certain biologics can influence supplier selection and encourage regionalization of supply chains. In turn, this compels manufacturers and purchasers to re-evaluate contract structures, inventory strategies, and local production investments to mitigate cost exposure and maintain continuity of care.These developments also affect the economics of novel therapeutic modalities that rely on specialized manufacturing processes or cold-chain logistics. For developers, higher cross-border transaction costs can lengthen commercialization timelines and intensify the capital required to scale manufacturing. For health systems, tariffs can increase procurement complexity and accentuate inequities in access if regional suppliers cannot meet demand. As a result, stakeholders will need to balance near-term cost pressures with long-term resilience initiatives, considering options such as strategic partnerships with regional manufacturers, dual-sourcing agreements, and targeted investments in domestic production capacity to safeguard supply for critical agents.
Granular segmentation-driven insights that distinguish therapeutic modalities, pathogen priorities, clinical indications, and end-use settings to guide R&D and access strategies
Segment-level insights reveal heterogeneous opportunity and risk profiles across classes of therapeutic offerings and clinical contexts, and this heterogeneity should guide prioritization of R&D, commercialization, and stewardship efforts. Offering-based distinctions matter because combination therapies, immunomodulators, monoclonal antibodies, and phage-based approaches carry different development pathways, regulatory expectations, manufacturing complexities, and routes to market compared with traditional drugs. Within conventional drugs, chemical classes such as aminoglycosides, beta-lactams, fluoroquinolones, glycopeptides, macrolides, oxazolidinones, polymyxins, sulfonamides, and tetracyclines each present unique resistance mechanisms, safety profiles, and clinical roles that influence prescribing patterns and lifecycle management.Pathogen-specific segmentation is equally consequential; pathogens such as Acinetobacter baumannii, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pneumoniae differ in genetic adaptability, transmission dynamics, and clinical impact, shaping both therapeutic prioritization and diagnostic needs. Infection-type segmentation-covering acute bacterial skin and skin structure infections, bloodstream infections, Clostridioides difficile infection, community-acquired bacterial pneumonia, and complicated urinary tract infections-maps onto distinct care settings, outcome metrics, and reimbursement models. Finally, end-use settings including ambulatory care centers, diagnostic laboratories, hospitals and clinics, and research institutes determine how therapies and diagnostics are deployed operationally and financially. Integrating these dimensions provides a granular view that supports targeted product development and more precise market access strategies.
A regional lens on resistance responses that illuminates differences in policy, procurement, manufacturing capacity, and adoption pathways across global markets
Regional dynamics shape both the clinical burden of antimicrobial resistance and the ecosystem responses available to mitigate its effects, underscoring that geography remains a primary determinant of policy, procurement, and innovation adoption. In the Americas, healthcare systems vary widely in terms of payer structures, diagnostic penetration, and manufacturing presence, which influences how rapidly new modalities can be adopted and how procurement strategies evolve to ensure supply continuity. Stakeholders in this region often weigh centralized reimbursement pathways against the need for rapid innovation uptake in high-acuity settings.The Europe, Middle East & Africa region encompasses a broad range of capacity and regulatory sophistication, with some markets demonstrating advanced stewardship programs and robust regulatory alignment, while others face infrastructural and access challenges that constrain adoption. Cross-border collaboration and harmonized regulatory approaches in parts of this region create opportunities for coordinated trials and pooled procurement, but supply chain vulnerabilities remain a critical concern. The Asia-Pacific region features significant manufacturing capacity and a growing presence in advanced therapeutics, coupled with diverse healthcare financing arrangements. This region often serves as both a production hub and a major demand center, making policy shifts and trade measures particularly influential on global supply dynamics. Understanding these regional nuances is essential for tailoring engagement models, evidence generation, and manufacturing strategies.
Corporate strategic moves and collaborative models that are aligning therapeutic innovation, diagnostic integration, and manufacturing resilience to address resistance challenges
Leading organizations across biopharma, diagnostics, and contract manufacturing are responding to antibiotic resistance through diversified portfolios, strategic collaborations, and investments in next-generation modalities. Companies are increasingly pairing therapeutic development with companion or stand-alone diagnostics to strengthen value propositions and support stewardship objectives. Strategic alliances between innovators and large-scale manufacturers aim to resolve scale-up challenges for biologics and phage therapies, while public-private partnerships continue to play a role in de-risking early-stage development and ensuring clinical trial capacity.Providers and payers are also shaping the competitive landscape by demanding robust evidence of clinical effectiveness and cost impacts, which influences go-to-market strategies and commercial evidence plans. Diagnostic firms that can demonstrate rapid, accurate pathogen identification are finding stronger traction, especially when integration with electronic health records and stewardship protocols is feasible. Meanwhile, contract development and manufacturing organizations that can offer flexible, compliant processes for both small molecules and biologics are attracting interest from developers seeking to shorten time-to-market. Together, these corporate strategies illustrate an ecosystem-level pivot toward integrated solutions that align therapeutic innovation with diagnostics, manufacturing resilience, and payer-facing outcomes evidence.
Actionable strategic imperatives for leaders that balance supply resilience, diagnostic-therapeutic integration, evidence generation, and collaborative policy engagement
Industry leaders must adopt a multifaceted approach that balances near-term operational stability with long-term innovation investments to tackle antibiotic resistance effectively. First, prioritizing integrated development programs that combine therapeutic candidates with rapid diagnostics will strengthen clinical value propositions and support stewardship goals, thereby improving adoption prospects in diverse care settings. Second, pursuing flexible manufacturing agreements and regional partnerships can reduce exposure to trade disruptions and accelerate local availability of critical therapeutics; this includes evaluating alliances with contract manufacturers and capacity-sharing arrangements.Third, investing in robust real-world evidence generation and health-economic modeling will aid in negotiating reimbursement and in demonstrating the broader system-level benefits of novel interventions. Fourth, engaging proactively with regulators and payers through adaptive evidence pathways can streamline approvals and reimbursement while ensuring that safety and effectiveness benchmarks are met. Finally, fostering cross-sector collaborations that include public health agencies, academic centers, and patient groups will amplify impact by aligning incentives for stewardship, surveillance, and access. Implementing these actions in a coordinated manner will improve resilience, de-risk commercial launches, and maximize clinical benefits across settings.
Methodological transparency and multi-source synthesis providing a robust foundation for actionable insights without relying on speculative projections
The research underpinning this executive summary employed a mixed-methods approach that synthesized peer-reviewed clinical literature, regulatory guidance updates, public health surveillance data, and expert interviews with stakeholders across industry, clinical practice, and policy domains. Qualitative synthesis prioritized evidence on therapeutic mechanisms, resistance pathways, and clinical outcomes, while thematic analysis of stakeholder interviews identified practical barriers to adoption and operational considerations for manufacturing and distribution.To ensure robustness, methodological triangulation was applied by cross-referencing regulatory developments with observed shifts in procurement practices and published clinical trial outcomes. Where appropriate, comparative analysis across therapeutic classes and pathogen types illuminated differential development and adoption trajectories. The methodology emphasized transparency regarding source provenance and analytic assumptions, and sensitivity analyses were used to examine how alternative supply chain and policy scenarios could influence strategic choices. This methodological foundation supports the report’s focus on actionable insights rather than predictive quantification, and it aims to be replicable for subsequent deep dives or bespoke client engagements.
A synthesis of strategic priorities that underscores the need for coordinated innovation, manufacturing agility, and policy-aligned stewardship to sustain clinical efficacy
Antibiotic resistance demands sustained, coordinated action that aligns scientific innovation with pragmatic operational and policy responses. Across therapeutic classes and clinical contexts, the most promising pathways combine targeted biologic and phage approaches with enhanced diagnostics and evidence generation to support stewardship and reimbursement. At the same time, supply chain resilience and adaptive regulatory engagement are essential to ensure that therapeutic advances translate into accessible, reliable care.Moving forward, success will depend on the ability of stakeholders to integrate cross-disciplinary expertise, invest in manufacturing and distribution agility, and craft reimbursement strategies that reflect clinical value beyond unit pricing. Collaborative models that bridge public health priorities with commercial incentives will be critical to sustaining momentum. In sum, a coordinated ecosystem response-anchored in rigorous evidence, strategic partnerships, and operational foresight-offers the best prospect for preserving therapeutic efficacy while enabling responsible innovation.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Antibiotic Resistance Market
Companies Mentioned
- Abbott Laboratories
- AbbVie Inc.
- ANTABIO
- AstraZeneca plc
- Basilea Pharmaceutica Ltd
- Bayer AG
- bioMérieux S.A.
- BioVersys AG
- C.H. Boehringer Sohn AG & Co. KG
- Cipla Limited
- Cumberland Pharmaceuticals
- Day Zero Diagnostics Inc.
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd
- GlaxoSmithKline plc
- Glox Theraputics Ltd
- Hikma Pharmaceuticals PLC
- Innoviva, Inc.
- Johnson & Johnson Services, Inc.
- Melinta Therapeutics LLC
- Merck & Co., Inc.
- Novo Holdings A/S
- Paratek Pharmaceuticals, Inc.
- Pfizer Inc.
- Sandoz AG
- Seres Therapeutics, Inc.
- Shionogi & Co., Ltd.
- Teva Pharmaceutical Industries Ltd.
- Theravance Biopharma, Inc.
- Wockhardt
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 194 |
| Published | January 2026 |
| Forecast Period | 2025 - 2030 |
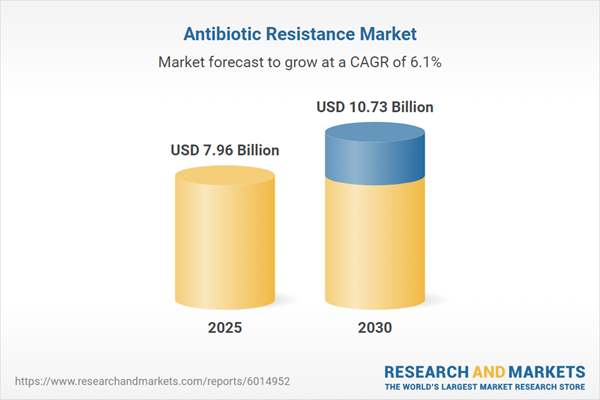
| Estimated Market Value ( USD | $ 7.96 Billion |
| Forecasted Market Value ( USD | $ 10.73 Billion |
| Compound Annual Growth Rate | 6.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |