Global Aortic Valves Market - Key Trends & Drivers Summarized
Why Are Aortic Valves Central to the Management of Structural Heart Disease in Aging Populations?
Aortic valves play a vital role in regulating unidirectional blood flow from the left ventricle to the aorta, and their dysfunction - typically stenosis or regurgitation - leads to significant cardiac morbidity and mortality. Aortic valve replacement (AVR), through either surgical or transcatheter approaches, has become a cornerstone intervention in structural heart disease, particularly among elderly patients with degenerative valve conditions. With the global burden of aortic stenosis rising in parallel with aging demographics, aortic valves are increasingly viewed as life-saving implants for restoring cardiac function, relieving symptoms, and improving survival rates.Historically, surgical aortic valve replacement (SAVR) was the standard of care, requiring open-heart surgery with cardiopulmonary bypass. However, the advent of transcatheter aortic valve replacement (TAVR) - a minimally invasive approach - has transformed treatment paradigms, expanding access to patients previously deemed inoperable or high-risk for surgery. TAVR's use has since expanded to intermediate and low-risk groups, reinforcing its role as a first-line therapy across broader patient cohorts. This shift is being fueled by favorable clinical trial data demonstrating comparable or superior outcomes relative to SAVR in appropriate candidates.
Beyond stenosis, aortic valve interventions are gaining ground in the management of bicuspid valve disease, valve-in-valve procedures for degenerated bioprostheses, and combined valvular pathologies requiring multi-valve interventions. The growing prevalence of heart failure, calcific valve degeneration, and increased screening in asymptomatic patients is further driving procedural volume. As life expectancy increases and cardiovascular disease remains a global health priority, aortic valve replacement continues to serve as a critical intervention in contemporary cardiology.
How Are Device Innovations and Procedural Technologies Enhancing Valve Performance and Patient Outcomes?
Innovations in valve design are improving hemodynamic performance, durability, and implantability of both surgical and transcatheter aortic valves. Bioprosthetic valves - constructed from bovine or porcine pericardial tissue - are now treated with anti-calcification agents and mounted on flexible frames to reduce immune response and extend lifespan. New-generation TAVR systems offer repositionable and retrievable features, lower-profile delivery catheters, and enhanced radial strength for secure anchoring, even in anatomically challenging cases such as severely calcified or bicuspid aortic roots.Advancements in pre-procedural imaging, especially multidetector CT and 3D echocardiography, have enabled more precise patient selection, annular sizing, and procedural planning. Integration of imaging with digital navigation platforms and real-time fluoroscopic guidance has minimized paravalvular leak, vascular injury, and conduction disturbances. In addition, intraprocedural innovations such as cerebral embolic protection devices and conscious sedation protocols are improving safety profiles and enabling shorter recovery times.
Durability remains a central focus of innovation, particularly as younger and lower-risk patients undergo TAVR. Ongoing trials are assessing leaflet longevity, structural valve deterioration, and thrombogenicity to support long-term adoption. Polymer-based leaflet designs, tissue-engineered scaffolds, and anti-thrombotic coatings are being explored to extend valve life beyond current bioprosthetic limits. Meanwhile, efforts to standardize valve-in-valve procedures, optimize hemodynamics in small annuli, and reduce pacemaker dependency are pushing the next frontier in device evolution.
Which Patient Segments and Regional Markets Are Fueling Demand for Aortic Valve Interventions?
The primary drivers of aortic valve replacement remain elderly patients with symptomatic severe aortic stenosis, particularly in North America and Western Europe where diagnostic access, procedural infrastructure, and reimbursement models are well-established. However, the increasing use of echocardiographic screening and growing awareness of heart valve disease are expanding referrals even among asymptomatic patients and moderate-risk groups. TAVR is increasingly preferred for frail patients, those with prior cardiac surgery, and those with anatomical complexities that increase open-surgery risk.Younger patients and those with bicuspid valves are emerging as an important focus, particularly in markets where average age at diagnosis is lower or where SAVR remains the default due to reimbursement constraints. The valve-in-valve segment is also growing rapidly as the first wave of bioprosthetic implants begins to degenerate. This is creating a new layer of demand for redo TAVR procedures, supported by growing evidence of safety and efficacy in reinterventions.
Geographically, North America holds the largest market share, followed by Europe, driven by high procedural volumes, advanced healthcare infrastructure, and established training ecosystems. Asia-Pacific represents the fastest-growing region due to expanding healthcare access, increasing awareness of valvular heart disease, and rapid growth in private cardiovascular centers across China, India, Japan, and Southeast Asia. Meanwhile, adoption in Latin America, the Middle East, and parts of Africa is supported by public-private partnerships, medical tourism, and growing investment in tertiary cardiovascular care infrastructure.
How Are Regulatory Developments, Value-Based Care Models, and Clinical Evidence Influencing Market Expansion?
The regulatory landscape for aortic valve devices is evolving rapidly, with authorities accelerating approval pathways for TAVR systems through priority review, breakthrough designation, and reliance on global clinical data. Agencies such as the FDA and EMA have expanded device indications from high-risk to intermediate- and low-risk patients, driving broader procedural eligibility and insurance coverage. Post-market surveillance and registries - such as TVT in the U.S. and NOTION in Europe - are providing long-term outcomes data that support ongoing reimbursement and guideline inclusion.As healthcare systems adopt value-based models, cost-effectiveness and quality-of-life improvements are becoming pivotal to technology adoption. Numerous health economic studies have shown that TAVR, despite higher upfront costs, offers long-term value through reduced rehospitalization, improved functional outcomes, and lower mortality in eligible populations. Hospitals are increasingly selecting valve platforms based on procedure success rates, reintervention risk, and compatibility with evolving treatment protocols across multi-disciplinary heart teams.
Clinical guidelines from societies such as the ESC, AHA/ACC, and JCS are incorporating TAVR into mainstream recommendations for a broader patient population. Simultaneously, global training initiatives and cross-border knowledge exchange are supporting operator proficiency and standardization of care. As procedural outcomes improve and stakeholders demand data-backed decision-making, clinical evidence will remain a cornerstone of market growth and strategic differentiation among device manufacturers.
What Are the Factors Driving Growth in the Aortic Valves Market?
The aortic valves market is experiencing robust growth, fueled by the global rise in valvular heart disease, expanded eligibility for minimally invasive interventions, and the continuous shift toward patient-centered, evidence-based cardiovascular care. Key drivers include aging populations, increased screening, evolving treatment guidelines, and growing comfort with TAVR in younger and lower-risk cohorts. The transition from surgical to catheter-based interventions is particularly transformative in terms of procedural volume and accessibility.Technological differentiation, long-term durability, and clinical versatility are becoming critical to market competitiveness as stakeholders demand personalized, data-driven care pathways. Meanwhile, market expansion is supported by rising investment in hybrid ORs, specialized heart valve centers, and cross-functional cardiovascular teams trained in advanced valve therapies. As global health systems prioritize efficient, minimally invasive, and high-value care, aortic valve technologies are emerging as essential tools in structural heart disease management.
Looking forward, the evolution of the aortic valves market will depend on how effectively the next generation of devices balances performance, durability, and affordability across diverse healthcare settings. As precision cardiology converges with minimally invasive technologies, could aortic valves represent the central node of a global shift toward preventative and restorative heart care?
Report Scope
The report analyzes the Aortic Valves market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Valve Type (Mechanical Valves, Biological Valves); End-Use (Hospitals, Ambulatory Surgery Centers, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Mechanical Valves segment, which is expected to reach US$5.8 Billion by 2030 with a CAGR of a 7.1%. The Biological Valves segment is also set to grow at 10.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.7 Billion in 2024, and China, forecasted to grow at an impressive 12.6% CAGR to reach $2.1 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Aortic Valves Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Aortic Valves Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Aortic Valves Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Altura Medical Inc., Aptus Endosystems Inc., Artivion Inc., B. Braun Melsungen AG and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Aortic Valves market report include:
- Abbott Laboratories
- Anteris Technologies Ltd.
- Artivion Inc.
- AutoTissue GmbH
- Boston Scientific Corporation
- CarboMedics Inc.
- Cardiac Dimensions Inc.
- Cardiosolutions Inc.
- Corcym Inc.
- CryoLife Inc.
- Edwards Lifesciences
- Endovalve Inc.
- Foldax Inc.
- JenaValve Technology Inc.
- Leman Cardiovascular
- LivaNova PLC
- Medtentia AB
- Medtronic Plc
- MiCardia Corporation
- MitralSolutions Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Anteris Technologies Ltd.
- Artivion Inc.
- AutoTissue GmbH
- Boston Scientific Corporation
- CarboMedics Inc.
- Cardiac Dimensions Inc.
- Cardiosolutions Inc.
- Corcym Inc.
- CryoLife Inc.
- Edwards Lifesciences
- Endovalve Inc.
- Foldax Inc.
- JenaValve Technology Inc.
- Leman Cardiovascular
- LivaNova PLC
- Medtentia AB
- Medtronic Plc
- MiCardia Corporation
- MitralSolutions Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 268 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
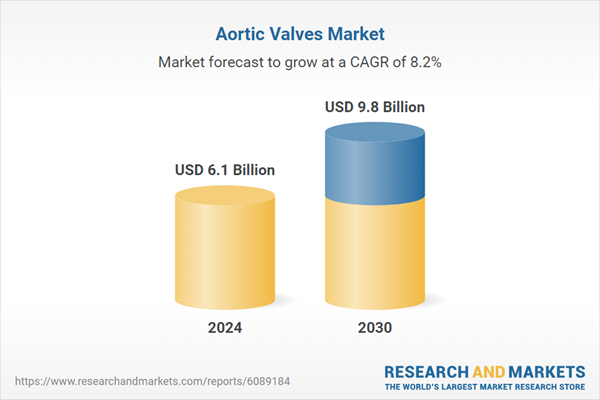
| Estimated Market Value ( USD | $ 6.1 Billion |
| Forecasted Market Value ( USD | $ 9.8 Billion |
| Compound Annual Growth Rate | 8.2% |
| Regions Covered | Global |