Rising Need for AI in Consent Management Fuels the Asia Pacific Antibody Drug Conjugates Market
Rising cancer incidences are primarily leading to the increasing demand for innovative cancer treatments. As per the Global Cancer Observatory (GLOBOCAN) estimates published in 2020, there were 19.3 million cancer cases across the globe. China, and India were among the countries leading in cancer cases. The GLOBOCAN has also estimated that the cancer cases in India will be ~2.08 million by 2040, representing a rise of 57.5% from 2020. Similarly, as per the World Health Organization (WHO) data published in February 2022, ~10 million people have died from cancer. The most commonly seen cancer types were lung, breast, colon and rectum, prostate, and breast. The rising incidences of cancer have raised concerns worldwide. Below is the list of common cancer cases reported worldwide in 2020.Table 1. New Cancer Cases Worldwide, in 2020
Sr. No. Cancer Type Number of Cases (Million)
1 Stomach 1.09
2 Skin (non-melanoma) 1.2
3 Prostate 1.41
4 Colon and Rectum 1.93
5 Lung 2.21
6 Breast 2.26
Cancer is now a lifestyle disease commonly seen among people with unhealthy food habits and who are physically inactive. Also, the consumption of alcohol and tobacco adds up to the risk of developing cancer. Chronic infectious diseases in low- and middle-income countries can further aggravate the cancer risk factors. According to the WHO data published in February 2022, chronic infectious diseases such as human papillomavirus (HPV), Helicobacter pylori, hepatitis B virus, hepatitis C virus, and Epstein-Barr virus attributed to nearly 18% of the diagnosed in 2018. Also, promising results of ADCs are anticipated to accelerate their demand as an effective cancer treatment and propel the market growth.Asia Pacific Antibody Drug Conjugates Market Overview
The Asia Pacific antibody drug conjugates (ADCs) market is segmented into China, Japan, India, South Korea, Australia, and the Rest of Asia Pacific. The growth of the ADCs market is widely driven by company collaborations, growing incidences of cancer, and increasing clinical trials.The leading companies in Asia Pacific countries that are involved in developing ADCs have pipeline products to be launched in the market after completing the clinical trials. In June 2023, RemeGen Co., Ltd. signed an agreement with Innovent Biologics, Inc. to enter clinical trials and supply combination therapies TYVYT (sintilimab injection) with RC88, a novel mesothelin (MSLN)-targeting antibody-drug conjugate (ADC), or TYVYT with RC108, a novel c-Met-targeting ADC. These are claimed to be novel therapies to treat solid tumors. Under the agreement, RemeGen Co., Ltd will conduct Phase 1/2a clinical studies for TYVYT to evaluate the anti-tumor activity and safety. The company also has Distamab vedotin (RC48) +PD1 combination in the pipeline. The combination is evaluated in two phases for different cancer indications, first combination is in phase III, evaluated to treat urothelial cancer in and second is in phase II, evaluated to treat muscle invasive bladder and gastric cancer.
In addition, Japanese companies are introducing breakthrough products that are expected to dominate the ADCs market. In June 2021, Daiichi Sankyo Company, Limited, and AstraZeneca plc launched Enhertu-a novel class of ADCs developed to treat HER2-low breast cancer patients. Daiichi Sankyo Company, Limited reported a year-on-year sales growth of 156.6% in the first quarter of 2023 by combining the US and European sales. Such a huge growth in the company's sales has significantly contributed to the ADCs market size in the country and is estimated to grow exponentially in the coming years.
Asia Pacific Antibody Drug Conjugates Market Segmentation
The Asia Pacific antibody drug conjugates market is segmented based on technology, application, distribution channel, and country.Based on technology, the Asia Pacific antibody drug conjugates market antibody drug conjugates market is bifurcated into cleavable linker and non-cleavable linker. The cleavable linker segment held a larger share in 2022.
Based on application, the Asia Pacific antibody drug conjugates market antibody drug conjugates market is segmented into blood cancer, breast cancer, ovarian cancer, urothelial cancer, and others. The breast cancer segment held the largest share in 2022.
Based on distribution channel, the Asia Pacific antibody drug conjugates market antibody drug conjugates market is segmented into hospital pharmacies, retail pharmacies, and online pharmacies. The hospital pharmacies segment held the largest share in 2022.
Based on country, the Asia Pacific antibody drug conjugates market is categorized into Australia, China, India, Japan, South Korea, and the Rest of Asia Pacific. Japan dominated the Asia Pacific antibody drug conjugates market in 2022.
Pfizer Inc, Hoffmann-La Roche Ltd, GSK Plc, Gilead Sciences Inc, AstraZeneca Plc, Astellas Pharma Inc, RemeGen Co Ltd, and Takeda Pharmaceutical Co Ltd are some of the leading companies operating in the Asia Pacific antibody drug conjugates market.
Table of Contents
Companies Mentioned
- Pfizer Inc
- Hoffmann-La Roche Ltd
- GSK Plc
- Gilead Sciences Inc
- AstraZeneca Plc
- Astellas Pharma Inc
- RemeGen Co Ltd
- Takeda Pharmaceutical Co Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 101 |
| Published | March 2024 |
| Forecast Period | 2022 - 2030 |
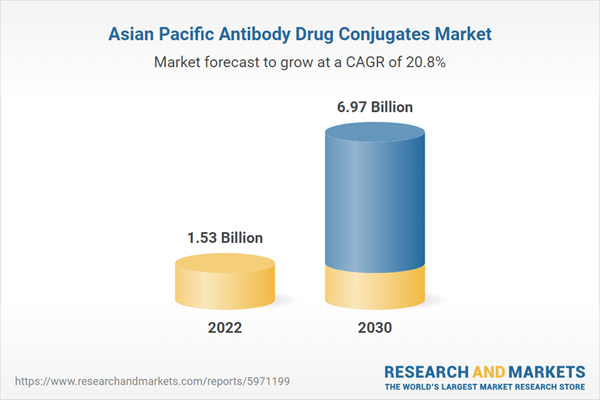
| Estimated Market Value in 2022 | 1.53 Billion |
| Forecasted Market Value by 2030 | 6.97 Billion |
| Compound Annual Growth Rate | 20.8% |
| Regions Covered | Asia Pacific |
| No. of Companies Mentioned | 8 |