Speak directly to the analyst to clarify any post sales queries you may have.
Unveiling the Strategic Importance of Atrial Septal Defect Management and Emerging Opportunities in Interventional Cardiology Devices
Atrial septal defect (ASD) represents a significant congenital cardiac anomaly characterized by an opening in the atrial septum, leading to altered hemodynamics, right heart enlargement, and potential complications such as arrhythmias and pulmonary hypertension. With advances in diagnostic imaging and minimally invasive closure techniques, management paradigms have shifted dramatically over the past decade. The evolving landscape encompasses a spectrum of interventional devices from occluders designed for catheter-based deployment to refined surgical patches, each offering unique clinical and economic profiles.This executive summary offers a holistic overview of the ASD market, synthesizing recent clinical advancements, regulatory trends, and competitive forces. It underscores the critical intersection of technological innovation, demographic shifts, and health policy developments shaping device adoption. By contextualizing these factors within the broader framework of cardiovascular care, the analysis prepares stakeholders to navigate emerging risks and capitalize on opportunities in product development, market access, and patient outcomes.
Examining Paradigm Shifts in Atrial Septal Defect Management Fueled by Technological Breakthroughs and Patient-Centric Innovations
The ASD treatment landscape has entered a transformative era driven by next-generation device architectures, digital health integration, and patient-centric protocols. Transitioning from conventional open-heart procedures to catheter-delivered occluders has not only reduced perioperative morbidity but also heralded a new focus on rapid recovery pathways and outpatient care models. Innovations in balloon-expandable and self-expanding occluder materials have enhanced conformability and reduced procedural time, reflecting a broader industry shift toward customizable, precision-engineered solutions.Simultaneously, advances in cardiac imaging systems and real-time hemodynamic monitoring are empowering interventional cardiologists to achieve greater procedural accuracy, resulting in higher closure rates and improved long-term outcomes. These technological breakthroughs are complemented by evolving clinical guidelines that emphasize early diagnosis and timely intervention, thereby expanding the eligible patient population. Taken together, these paradigm shifts are redefining value propositions for both providers and payers, setting the stage for continued growth and innovation in ASD care.
Assessing the Far-Reaching Effects of New United States Tariff Measures on Atrial Septal Defect Device Supply Chains and Cost Structures
The introduction of new United States tariff measures in 2025 has had a profound impact on the ASD device ecosystem, reshaping supply chain configurations and cost structures. Manufacturers reliant on imported cardiac catheterization equipment and specialized implantable devices have faced escalating input costs, leading to strategic recalibrations in procurement and sourcing. As a result, several device producers have accelerated efforts to localize critical components or establish domestic assembly lines to mitigate exposure to tariff fluctuations.Moreover, the ripple effects of increased levies have prompted payers and institutional purchasers to negotiate more aggressive pricing agreements and demand greater transparency in total cost of care. In response, leading companies are exploring alternative materials and streamlined manufacturing processes that maintain clinical performance while containing expenses. These adaptations, while initially driven by external fiscal pressures, are fostering long-term resilience and could ultimately catalyze regional manufacturing hubs. Consequently, market participants who proactively address tariff-related challenges will be better positioned to sustain competitive margins and ensure uninterrupted device availability.
Deep Dive into Market Segmentation Insights Revealing Nuanced Opportunities Across Devices Treatment Modalities Age Groups and Care Settings
A nuanced understanding of market segmentation is pivotal for identifying growth vectors in the ASD domain. Across product types, the cardiopulmonary bypass segment remains essential for complex surgical interventions, while closure devices-subdivided into occluders and patches-are driving momentum in minimally invasive settings. Within occluders, balloon-expandable and self-expanding technologies offer divergent profiles in terms of deliverability and patient suitability, whereas biologic and synthetic patches cater to varying preferences in tissue compatibility and long-term integrity. Diagnostic devices, encompassing cardiac catheterization equipment, advanced imaging systems, and echocardiography platforms, continue to underpin early detection and intraoperative guidance.When considering treatment modalities, interventional approaches are increasingly favored due to reduced hospital stays and faster recovery, yet non-interventional management retains a role in patients with contraindications or complex comorbidities. Age group analysis reveals distinct opportunities: adult cohorts benefit from streamlined percutaneous solutions, while pediatric subsegments from adolescent to neonatal require device customizations and tailored procedural techniques. Finally, adoption patterns among ambulatory surgical centers, specialized cardiac care facilities, and hospitals vary dramatically based on procedural volume, reimbursement frameworks, and infrastructure capabilities. This granular segmentation framework illuminates precisely where to focus innovation and resource allocation.
Charting Regional Dynamics and Growth Drivers Across the Americas Europe Middle East Africa and Asia Pacific in Atrial Septal Defect Care
Regional dynamics in ASD care are shaped by distinct demographic trends, healthcare infrastructures, and policy environments. In the Americas, high diagnostic rates and established reimbursement mechanisms have fostered widespread adoption of minimally invasive closure devices, prompting further investment in patient follow-up and outcome registries. Transitioning to Europe, the Middle East, and Africa, variability in access to advanced imaging and interventional expertise drives selective uptake, with tiered pricing strategies and public-private partnerships playing a crucial role in expanding procedural capacity. Meanwhile, rapid urbanization and growing healthcare expenditure in the Asia-Pacific region are catalyzing the penetration of novel device platforms, supported by localized clinical trials and regulatory harmonization efforts.Each regional cluster offers distinct challenges and prospects: while mature markets prioritize incremental device enhancements and value-based care integration, emerging economies emphasize cost-effective solutions, capacity building, and the establishment of center-of-excellence networks. Recognizing these divergent pathways enables stakeholders to tailor market entry strategies, optimize resource deployment, and foster sustainable growth across heterogeneous geographies.
Illuminating Leading Company Strategies and Competitive Landscapes Shaping the Future of Atrial Septal Defect Intervention Devices
Competitive landscapes in the ASD segment are characterized by a blend of established medtech leaders and agile innovators. Major corporations with extensive device portfolios are leveraging scale to support global clinical studies and invest in next-generation materials science. These entities often enjoy strong brand recognition and deep relationships with key opinion leaders, enabling them to influence clinical guidelines and secure favorable procurement contracts. At the same time, smaller specialized firms are disrupting the status quo by focusing on high-performance alloys, bioresorbable components, and proprietary delivery mechanisms that enhance procedural efficiency.Strategic partnerships between device manufacturers and digital health providers are emerging as a critical differentiator, facilitating remote monitoring, data analytics, and patient engagement initiatives that extend beyond the cath lab. Collaborations with academic institutions further bolster the innovation pipeline, ensuring a steady flow of novel concepts from bench to bedside. In this competitive context, success will hinge on the ability to integrate cross-functional expertise, demonstrate robust clinical and economic value, and navigate complex regulatory pathways efficiently.
Actionable Strategic Recommendations to Optimize Portfolio Development Stakeholder Engagement and Market Access in Atrial Septal Defect Therapies
Industry leaders can capitalize on emerging trends by prioritizing several strategic imperatives. First, accelerating the development of next-generation occluders with adaptive conformability and enhanced imaging compatibility will address clinician demand for precision and ease of use. Second, establishing integrated care models that combine device deployment with long-term patient monitoring and digital health solutions can differentiate offerings and support outcomes-based reimbursement frameworks. Third, forging alliances to localize manufacturing or secure strategic component supply agreements will mitigate tariff risks and stabilize cost structures.Additionally, tailoring engagement with payers, healthcare providers, and patient advocacy groups is essential to build consensus around value propositions and facilitate guideline adoption. Finally, investing in real-world evidence generation through post-market registries and collaborative research can substantiate safety, efficacy, and cost-effectiveness, thereby reinforcing market credibility. By executing these actionable initiatives, organizations will be well-positioned to lead in a competitive, rapidly evolving landscape.
Transparent Research Methodology Integrating Rigorous Data Collection Analytical Techniques and Multi-Source Validation for Robust Market Insights
This analysis is built upon a rigorous methodology integrating primary research, secondary data sources, and expert validation. Primary research involved interviews with interventional cardiologists, biomedical engineers, and health economics specialists to gain qualitative insights into clinical practices, device preferences, and reimbursement considerations. Secondary research leveraged peer-reviewed journals, regulatory publications, and publicly disclosed financial reports to quantify technology readiness and competitive positioning. Cross-referencing multiple data streams ensured consistency and uncovered emerging trends.Advanced analytical techniques, including scenario modeling and sensitivity analysis, were applied to assess the impact of tariff fluctuations, segmentation growth drivers, and regional adoption patterns. To validate findings, structured consultations were conducted with key opinion leaders and health policy advisors, providing real-world context and ensuring robustness. This transparent, multi-source approach delivers actionable intelligence while upholding the highest standards of data integrity and methodological rigor.
Concluding Perspectives Emphasizing Integrated Approaches and Future Directions in Atrial Septal Defect Innovation and Clinical Practice
In conclusion, the atrial septal defect landscape is undergoing rapid evolution fueled by technological innovation, shifting clinical paradigms, and an increasingly complex regulatory and tariff environment. Stakeholders who embrace precision-engineered devices, foster integrated care models, and adapt proactively to fiscal policy changes will capture significant value. Granular segmentation insights reveal targeted opportunities across product types, treatment modalities, age cohorts, and care settings, while regional analyses highlight the importance of context-specific strategies.To maintain leadership, organizations must continue investing in research and development, cultivate strategic partnerships, and generate compelling real-world evidence. By aligning internal capabilities with external trends, industry players can drive improved patient outcomes, secure market access, and achieve sustainable growth in a dynamic cardiac care ecosystem.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Atrial Septal Defect Market
Companies Mentioned
The key companies profiled in this Atrial Septal Defect market report include:- Abbott Laboratories
- Arjo AB
- ASAHI INTECC CO., LTD.
- Asklepion Pharmaceuticals, LLC
- atHeart Medical AG
- AtriCure, Inc
- B. Braun Medical Inc.
- Becton, Dickinson and Company
- Bio-tronik SE & Co. KG
- Boston Scientific Corporation
- Carag AG
- Cardia, Inc.
- Coherex Medical, Inc.
- Cook Medical, Inc
- Edwards Lifesciences
- GE HealthCare Technologies, Inc.
- Heart Medical Europe BV
- Johnson & Johnson Services, Inc.
- Kaneka Corporation
- Keystone Heart Ltd.
- Koninklijke Philips N.V.
- Lepu Medical Technology (Beijing) Co., Ltd
- Lifetech Scientific
- Medtronic PLC
- Microport Scientific Corporation
- Occlutech GmbH
- Osypka AG
- PFM Medical (Nit-Occlud ASD-R)
- Shanghai Shape Memory Alloy Co., Ltd.
- Siemens AG
- St. Jude Medical LLC
- Starway Medical Technology, Inc.
- Stryker Corporation
- Terumo Corporation
- Transcatheter Technologies GmbH
- Vascular Innovations
- Venus Medtech Hangzhou Inc
- Visionary Medtech Solutions
- W. L. Gore & Associates, Inc.
- Weigao Meidcal international Co., Ltd
Table Information
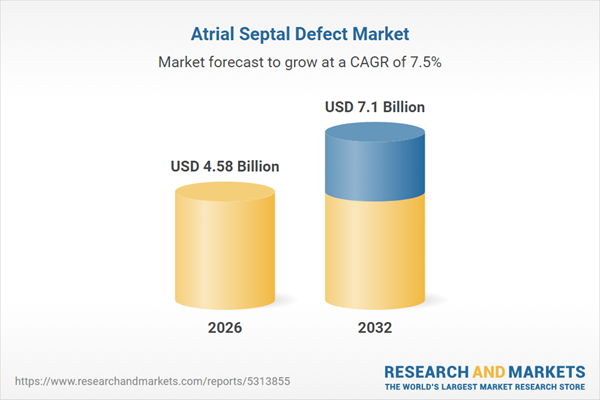
| Report Attribute | Details |
|---|---|
| No. of Pages | 195 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 4.58 Billion |
| Forecasted Market Value ( USD | $ 7.1 Billion |
| Compound Annual Growth Rate | 7.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 41 |