Global Benign Prostatic Hyperplasia (BPH) Drugs Market - Key Trends and Drivers Summarized
What Is Benign Prostatic Hyperplasia and How Are Drugs Used to Manage It?
Benign Prostatic Hyperplasia (BPH) is a common condition affecting older men, characterized by the non-cancerous enlargement of the prostate gland. This enlargement can lead to uncomfortable urinary symptoms, such as difficulty in starting urination, a weak urine stream, and the frequent need to urinate, especially at night. As the prostate enlarges, it presses against the urethra, obstructing the flow of urine and leading to potential complications such as urinary tract infections, bladder stones, and even kidney damage if left untreated. BPH is particularly prevalent in men over the age of 50, with the risk increasing with age. Managing BPH effectively is crucial not only for alleviating symptoms but also for improving the quality of life for those affected. As the condition becomes more common with the aging global population, the demand for effective treatments, including pharmacological options, is on the rise.How Do BPH Drugs Work and What Are the Available Treatment Options?
BPH drugs are designed to alleviate the symptoms associated with prostate enlargement by targeting different mechanisms involved in the condition. The two main classes of medications used to treat BPH are alpha-blockers and 5-alpha-reductase inhibitors. Alpha-blockers, such as tamsulosin and alfuzosin, work by relaxing the muscles in the prostate and bladder neck, making it easier to urinate. These drugs provide rapid relief from symptoms, usually within a few days. On the other hand, 5-alpha-reductase inhibitors, like finasteride and dutasteride, target the underlying cause of BPH by reducing the production of dihydrotestosterone (DHT), a hormone that contributes to prostate growth. While these medications take longer to show effects, they can reduce the size of the prostate over time and lower the risk of acute urinary retention or the need for surgery. Combination therapy, involving both alpha-blockers and 5-alpha-reductase inhibitors, is also commonly prescribed for more severe cases of BPH, providing both immediate symptom relief and long-term management of the condition.Why Is There a Growing Demand for BPH Drugs?
The demand for BPH drugs is increasing globally due to several factors, including the aging population and the rising awareness of prostate health issues. As the global population continues to age, the prevalence of BPH is expected to rise significantly, leading to greater demand for effective medical treatments. Additionally, increasing awareness and improved healthcare access are encouraging more men to seek treatment for urinary symptoms associated with BPH, rather than tolerating them as an inevitable part of aging. Advances in drug development are also contributing to the growing demand, as newer medications with fewer side effects and improved efficacy become available. Furthermore, the trend towards personalized medicine is leading to more tailored treatment approaches, where drug therapies are selected based on the patient's specific symptoms, prostate size, and overall health. These developments are driving the adoption of BPH drugs as an essential part of managing the condition and improving patients' quality of life.What Is Fueling the Expansion of the BPH Drugs Market?
The growth in the BPH drugs market is driven by several factors, including the increasing prevalence of BPH due to the aging global population, which is leading to a higher demand for effective treatment options. Technological advancements in pharmaceutical research are resulting in the development of more targeted and effective drugs with fewer side effects, further propelling market growth. The expanding healthcare infrastructure in emerging markets is also contributing to increased access to BPH treatments, leading to a broader adoption of these drugs. Additionally, the rising awareness among men regarding the importance of early diagnosis and treatment of BPH is driving market expansion, as more individuals seek medical intervention at the onset of symptoms. The trend towards combination therapies, which offer comprehensive symptom relief and long-term management, is another significant factor supporting market growth. As these factors continue to influence the landscape, the BPH drugs market is expected to see sustained growth, driven by ongoing innovations and the increasing need for effective management of this common condition.Report Scope
The report analyzes the Benign Prostatic Hyperplasia (BPH) Drugs market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Therapeutic Class (Alpha-Blockers, 5-ARIs, PDE-5 Inhibitors, Other Therapeutic Classes).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Alpha-Blockers segment, which is expected to reach US$7.4 Billion by 2030 with a CAGR of 6%. The 5-ARIs segment is also set to grow at 5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $2.2 Billion in 2024, and China, forecasted to grow at an impressive 5.4% CAGR to reach $1.7 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Benign Prostatic Hyperplasia (BPH) Drugs Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Benign Prostatic Hyperplasia (BPH) Drugs Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Benign Prostatic Hyperplasia (BPH) Drugs Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ASKA Pharmaceutical Co., Ltd., Astellas Pharma, Inc., Eisai Co., Ltd., Eli Lilly and Company, Netmeds Marketplace Ltd. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 18 companies featured in this Benign Prostatic Hyperplasia (BPH) Drugs market report include:
- ASKA Pharmaceutical Co., Ltd.
- Astellas Pharma, Inc.
- Eisai Co., Ltd.
- Eli Lilly and Company
- Netmeds Marketplace Ltd.
- Stafford Pharmacy & Home Healthcare
- Synthon International Holding B.V.
- Teva Pharmaceutical Industries Ltd.
- Urologix, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- ASKA Pharmaceutical Co., Ltd.
- Astellas Pharma, Inc.
- Eisai Co., Ltd.
- Eli Lilly and Company
- Netmeds Marketplace Ltd.
- Stafford Pharmacy & Home Healthcare
- Synthon International Holding B.V.
- Teva Pharmaceutical Industries Ltd.
- Urologix, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
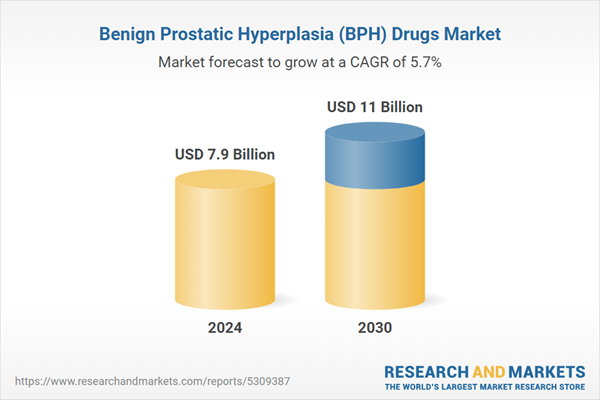
| Estimated Market Value ( USD | $ 7.9 Billion |
| Forecasted Market Value ( USD | $ 11 Billion |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |