Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
A major obstacle hindering market progression is the substantial capital investment and stringent regulatory oversight needed during the development stage, resulting in extended timelines and higher failure rates. As reported by the International Federation of Pharmaceutical Manufacturers and Associations in 2024, biologics represented roughly 50% of the 12,700 distinct drugs currently in global clinical development. This significant share underscores the industry's dependence on this sophisticated therapeutic class, notwithstanding the considerable operational and financial challenges associated with it.
Market Drivers
The incorporation of Artificial Intelligence (AI) and Machine Learning (ML) is transforming the Global Biologics Drug Discovery Market by optimizing the detection and refinement of intricate therapeutic candidates. By utilizing sophisticated algorithms for de novo protein design and predictive modeling, pharmaceutical firms can considerably reduce the high failure rates typically linked to early-phase biologics creation. This technological convergence facilitates rapid genomic data analysis and exact monoclonal antibody engineering, speeding up the progression from target validation to clinical testing. As noted in a September 2025 press release, Sanofi pledged an extra $625 million to its venture capital division to hasten investments in digital health and artificial intelligence, highlighting the industry's strategic shift toward computational discovery methods.Increasing investment and expenditure in biopharmaceutical R&D act as a fundamental catalyst, driving the ongoing investigation of advanced biologics like bispecific antibodies and gene therapies. The elevated costs associated with developing biologics, driven by complex manufacturing needs and strict safety testing, necessitate continuous capital funding to maintain a strong pipeline of new treatments. For instance, Johnson & Johnson reported in its January 2025 results for the full year 2024 a total R&D spend of $17.4 billion, demonstrating the massive financial dedication needed to achieve therapeutic innovations. This investment landscape directly bolsters regulatory productivity; the FDA's 'Novel Drug Approvals for 2024' report from January 2025 noted the approval of 50 new drugs, emphasizing how these significant R&D expenditures successfully translate into authorized therapies.
Market Challenges
The intense capital requirements and strict regulatory oversight inherent in the development process serve as major impediments to the growth of the Global Biologics Drug Discovery Market. These obstacles force companies to direct massive financial assets into research and development, frequently shifting funds away from pipeline diversification efforts. The rigorous standards mandated by health agencies require extensive safety evaluations and clinical trials, which extend development schedules and heighten the risk of failure in later stages. Consequently, smaller biotech enterprises often face difficulties maintaining operations without external partnerships, resulting in market consolidation that hampers the general rate of product introduction and innovation.The scale of these financial hurdles heavily affects the industry's capacity to expand operations and deliver new treatments to patients effectively. This strain is evident in the massive investments necessary to maneuver through the complicated discovery and development terrain. According to data from the European Federation of Pharmaceutical Industries and Associations in 2024, the pharmaceutical sector allocated roughly €55 billion to research and development across Europe. This substantial financial outlay highlights the severe pressure on market participants to control expenses while meeting compliance mandates, a situation that directly limits the overall growth potential of the global market.
Market Trends
The emergence of Antibody-Drug Conjugates (ADCs) within oncology pipelines is radically altering the Global Biologics Drug Discovery Market, prompting a wave of strategic acquisitions aimed at securing proprietary payload and linker technologies. Pharmaceutical firms are increasingly prioritizing these precise therapies to enhance treatment efficacy while reducing systemic toxicity, a transition that has triggered high-value consolidation in the industry to obtain proven platforms. This vigorous chase for next-generation assets is illustrated by leading players merging specialized skills to strengthen their cancer portfolios. For example, Johnson & Johnson announced in a March 2024 press release the completion of its acquisition of Ambrx Biopharma for an equity value of roughly $2.0 billion, a strategic action intended to fast-track the creation of precision antibody-drug conjugates for treating solid tumors and prostate cancer.The growth of outsourcing partnerships with Contract Research Organizations (CROs) has emerged as a prevailing operational trend, as the intricacy of biologic modalities demands specialized external knowledge. Innovators are shifting away from complete vertical integration, choosing instead to cooperate with comprehensive service providers that possess advanced skills in functional screening, cell line development, and scalable production. Relying on these strategic alliances enables companies to overcome technical obstacles and hasten market entry without shouldering the entire weight of capital-heavy infrastructure. As stated in Samsung Biologics' financial results for the fourth quarter and fiscal year 2024 released in January 2025, the firm recorded a record consolidated revenue of KRW 4.55 trillion, a 23% rise attributed to the successful launch of dedicated services for complex biologics and the increased capacity of its manufacturing facilities.
Key Players Profiled in the Biologics Drug Discovery Market
- AbbVie, Inc.
- Astellas Pharma, Inc.
- AstraZeneca PLC
- Bayer AG
- Bicon Ltd.
- Boehringer Ingelheim International GmbH
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd.
- Gilead Sciences, Inc.
- Catalent, Inc.
Report Scope
In this report, the Global Biologics Drug Discovery Market has been segmented into the following categories:Biologics Drug Discovery Market, by Type:
- Monoclonal Antibodies
- Recombinant Proteins
- Others
Biologics Drug Discovery Market, by Method:
- Target Identification/ validation
- Hit Generation/ Validation
- Lead Identification
- Lead Optimization
Biologics Drug Discovery Market, by Manufacture Type:
- In-House
- Outsourced
Biologics Drug Discovery Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Biologics Drug Discovery Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Biologics Drug Discovery market report include:- AbbVie, Inc.
- Astellas Pharma, Inc.
- AstraZeneca PLC
- Bayer AG
- Bicon Ltd.
- Boehringer Ingelheim International GmbH
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd.
- Gilead Sciences, Inc.
- Catalent, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
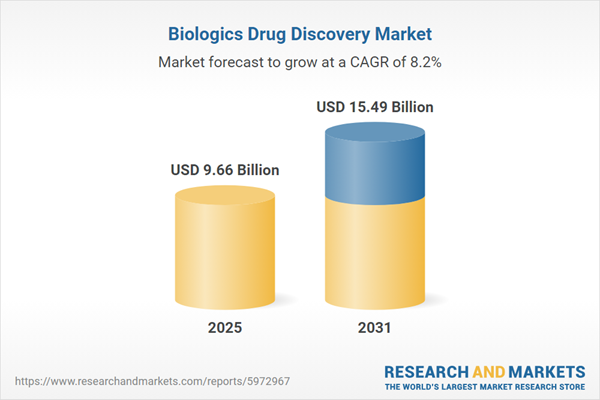
| Estimated Market Value ( USD | $ 9.66 Billion |
| Forecasted Market Value ( USD | $ 15.49 Billion |
| Compound Annual Growth Rate | 8.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |