Speak directly to the analyst to clarify any post sales queries you may have.
Strategic overview of the evolving botulinum toxins market as it shifts from niche therapy to multi-dimensional global platform
Botulinum toxins have evolved from a niche therapeutic intervention into a central pillar of both aesthetic medicine and multiple clinical specialties. Initially recognized for their neuromuscular blocking properties, these agents now sit at the intersection of cosmetic demand, chronic disease management, and innovation in minimally invasive procedures. The market today is shaped by a diverse array of stakeholders, including pharmaceutical originators, biosimilar challengers, device companies, healthcare providers, and an increasingly informed end user base.This evolution is unfolding against a backdrop of shifting patient expectations and demographic realities. Aging populations in developed economies, coupled with a growing middle class in emerging markets, are fueling interest in non-surgical solutions that offer visible results with limited downtime. At the same time, clinicians continue to expand the medical role of botulinum toxins across conditions such as muscle spasticity, dystonias, chronic migraine, hyperhidrosis, and pain-related disorders, reinforcing their value beyond purely aesthetic outcomes.
Parallel to these clinical and consumer forces, the regulatory and competitive environments have become more complex. Authorities are tightening oversight of product quality, supply chain integrity, and promotional practices, while high brand awareness around leading toxin products is encouraging both premium and value-oriented strategies. This combination of demand diversification, therapeutic expansion, and competitive intensity makes a structured understanding of the botulinum toxins landscape indispensable for any organization seeking to allocate capital, shape portfolios, or refine go-to-market approaches.
Within this context, stakeholders need a holistic perspective that integrates scientific progress, patient and provider behavior, policy developments, and geographic nuances. The following analysis examines how transformative shifts, policy headwinds such as United States tariffs scheduled for 2025, and fine-grained segmentation and regional dynamics are collectively redefining opportunity and risk in this market.
Transformational forces redefining botulinum toxin demand, innovation pathways, and competitive dynamics across indications
The landscape for botulinum toxins is being reshaped by several transformative forces that extend well beyond traditional cosmetic usage. One of the most significant shifts is the normalization of injectable treatments as routine personal maintenance rather than occasional luxury. This cultural move, amplified by social media, teledermatology, and provider-led education, is lowering psychological barriers to treatment and driving earlier adoption among younger demographics looking for preventive care rather than corrective interventions. As aesthetic procedures become more mainstream, providers are emphasizing natural-looking outcomes and personalized dosing strategies, prompting manufacturers to differentiate on precision, predictability, and duration of effect.Simultaneously, the clinical role of botulinum toxins continues to broaden as evidence accumulates across neurology, rehabilitation medicine, gastroenterology, ophthalmology, and pain management. New protocols for muscle spasms and paralysis, focal dystonias, spasticity after stroke or spinal cord injury, and certain gastrointestinal disorders are deepening uptake in hospital and specialty clinic settings. There is also sustained interest in off-label uses related to chronic pain syndromes, pelvic floor dysfunction, and even mood disorders, although regulatory approvals lag behind clinical experimentation in some of these areas. This expanding therapeutic footprint is incentivizing investment in formulation science, delivery technologies, and long-acting variants.
Innovation is another critical axis of transformation. Companies are refining botulinum toxin type A and type B molecules to optimize onset, spread, and duration, as well as to reduce immunogenicity risks. Ready-to-use liquid formulations are emerging alongside traditional lyophilized powders to streamline preparation, minimize dosing errors, and reduce procedural time. In parallel, advances in injection guidance, such as ultrasound or electromyography-assisted placement for complex neuromuscular indications, are making treatments safer and more effective while enabling providers with varying levels of experience to deliver consistent results.
Digitalization is redefining how the market operates across the value chain. Online appointment platforms, social media marketing, and virtual consultations are helping aesthetic practices and medical spas reach new patient segments and increase retention. At the same time, remote monitoring tools and electronic health records make it easier for hospitals and clinics to track patient outcomes and refine injection regimens over multiple cycles. This shift to data-informed practice is raising expectations for real-world evidence from manufacturers, who must demonstrate value not only in controlled trials but in routine clinical workflows.
Regulatory and competitive dynamics are also undergoing a notable shift. Several markets are seeing greater scrutiny of adverse event reporting, product traceability, and counterfeit or grey-market toxins. As branded products remain dominant in many countries, interest is growing in alternative suppliers and biosimilar-style competitors, especially where price sensitivity is high. However, the complexity and safety profile of neurotoxin products create substantial barriers to entry, favoring companies that can demonstrate consistent manufacturing quality, robust pharmacovigilance, and strong educational support for providers. Collectively, these trends are transforming the market from a limited set of branded injectables into a more segmented ecosystem defined by indication, formulation, service model, and patient experience.
These shifts are not occurring in isolation; rather, they converge to redefine how value is created and captured. Organizations must now think in terms of integrated solutions that combine high-quality toxins, practitioner training, digital engagement, and tailored patient journeys. Those able to orchestrate these elements across aesthetic and medical domains are best positioned to secure durable advantage as the landscape continues to evolve.
Assessing how cumulative 2025 United States tariffs may reshape botulinum toxin supply chains, pricing, and strategic decisions
Trade policy is increasingly relevant for companies active in the botulinum toxins market, and the cumulative effect of United States tariffs planned for 2025 is poised to influence sourcing, pricing, and supply strategies. While exact tariff structures will depend on final policy decisions and product classifications, the clear trend toward tighter scrutiny of pharmaceutical and biologic imports has already prompted many manufacturers to reassess their exposure to cross-border cost volatility. For products or components sourced from jurisdictions subject to elevated duties, even modest percentage increases can compress margins or force difficult decisions on pricing.Manufacturers with production facilities or critical suppliers outside the United States may face higher landed costs for imports of finished toxins, intermediates, or key materials such as specialized vials and packaging. Some companies are exploring nearshoring or expanding domestic fill-finish capabilities to mitigate tariff risk and ensure continuity of supply to high-value segments like dermatology clinics and hospitals. This reconfiguration of supply chains can take time and capital, but it also provides opportunities to strengthen quality control, reduce lead times, and signal reliability to providers who are increasingly sensitive to disruption.
From a commercial standpoint, tariffs can exert upward pressure on prices in the United States, especially for products that lack close substitutes. However, intense competition in aesthetic applications, combined with patient price sensitivity and the critical role of toxins in practice revenue models, may limit how much of the added cost can be passed on. Providers may respond by optimizing dosing patterns, bundling procedures, or shifting volume toward products with more favorable net pricing after tariffs and rebates. In medical applications where reimbursement is more structured, manufacturers will need to balance payer negotiations with the need to preserve margins under new cost conditions.
The cumulative impact of 2025 tariffs also extends to research and development and portfolio strategy. Companies facing higher trade frictions may prioritize investments in products and formulations that can be produced within tariff-favorable regions or that target segments with greater pricing power, such as complex neurological indications or high-value pain management protocols. Conversely, suppliers with existing U.S.-based manufacturing may leverage their position in discussions with group purchasing organizations, integrated delivery networks, and large practice groups, emphasizing supply security and reduced exposure to global trade shocks.
Distributors and online retail platforms must likewise adjust. For offline retail channels serving medical spas and independent clinics, inventory planning will become more critical as cost and delivery timelines face greater uncertainty. Online retailers dealing in ancillary products and, where legal, direct-to-provider toxin supply will need to carefully monitor regulatory and customs developments to avoid delays and unexpected fees. The net result is that trade policy ceases to be a background factor and becomes a central consideration in sourcing, pricing, and contracting for botulinum toxin products destined for the U.S. market.
Ultimately, the cumulative impact of United States tariffs in 2025 is less about a single policy shock and more about a sustained elevation of trade-related risk. Companies that proactively scenario-plan, diversify supply bases, and align commercial strategies with evolving tariff regimes will be better equipped to preserve access, protect profitability, and maintain the confidence of healthcare professionals and patients who rely on stable toxin supply.
Deep segmentation insights reveal distinct clinical, operational, and commercial pathways within the botulinum toxins ecosystem
Understanding the botulinum toxins market requires close attention to its underlying segmentation, as each dimension reveals distinct patterns of demand, innovation, and competitive pressure. At the molecular level, the market is organized around botulinum toxin type A and botulinum toxin type B, with type A dominating many aesthetic and therapeutic use cases due to its well-characterized safety and efficacy profile. Type B plays a critical though more specialized role, particularly in patients who develop immunity or suboptimal responses to type A, and in selected neurological indications. This division underscores the importance of maintaining a diversified portfolio that can address varied clinical needs and immunogenicity profiles.Formulation choice between ready-to-use liquid and lyophilized powder exerts a strong influence on provider preferences and operational workflows. Traditional lyophilized powders require reconstitution, offering flexibility in dilution but adding steps, variability, and potential for preparation errors. Ready-to-use liquids, by contrast, streamline procedures, reduce preparation time, and enhance dosing consistency, attributes that are especially attractive in high-volume aesthetic practices and busy hospitals. As clinicians place greater emphasis on efficiency and standardization, demand is gradually tilting toward user-friendly liquid formats, though powders remain entrenched where cost considerations or long-standing familiarity prevail.
Mode of administration further differentiates market behavior, with intradermal and intramuscular injections serving distinct therapeutic and aesthetic purposes. Intramuscular delivery is central in classic applications such as facial wrinkle modulation, muscle spasm control, and management of spasticity and paralysis-related conditions. Intradermal injections, on the other hand, are gaining traction in indications like hyperhidrosis and in advanced aesthetic protocols sometimes referred to as microinjections that focus on skin quality, fine lines, and pore appearance. As technique-sensitive protocols proliferate, training and anatomical expertise become key value drivers, encouraging manufacturers to invest in comprehensive education and support.
Distribution channels split between offline retail and online retail highlight another layer of strategic choice. Offline channels encompass direct sales to hospitals and clinics, distribution through wholesalers, and supply to dermatology practices and medical spas. These pathways rely heavily on relationships, salesforce capabilities, and value-added services such as training and practice development support. Online retail, where regulations permit, offers convenience and broader access, particularly for consumables associated with toxin procedures and in some cases for the toxins themselves in business-to-business frameworks. The growth of digital purchasing platforms is prompting manufacturers and authorized distributors to refine channel governance to safeguard product authenticity and pricing integrity.
The most nuanced segmentation emerges when examining applications. Aesthetic applications encompass facial aesthetics, hyperhidrosis, and non-surgical facelifts, each with its own patient expectations and clinical techniques. Facial aesthetics remains a powerhouse, driven by demand for minimally invasive rejuvenation and preventative treatments among diverse age groups. Hyperhidrosis, while more medically oriented, often overlaps with lifestyle motivations and can significantly improve quality of life. Non-surgical facelifts reflect sophisticated multi-point injection strategies aimed at lifting and contouring without incisions, positioning toxins as part of comprehensive facial harmonization protocols. On the medical side, indications in gastrointestinal disorders, muscle spasms and paralysis, and pain management highlight the versatility of toxins as neuromodulators. These applications are grounded in rigorous clinical pathways, multidisciplinary care teams, and payer involvement, which together shape adoption patterns and reimbursement debates.
End user segmentation brings the picture into clearer focus. Dermatology clinics remain central to both facial aesthetics and certain skin-related medical uses, leveraging specialized expertise and loyal patient bases. Hospitals and clinics are crucial for more complex therapeutic indications, including severe muscle spasms, paralysis-related conditions, and some gastrointestinal disorders, where the need for multidisciplinary oversight and advanced imaging or monitoring capabilities is higher. Medical spas serve as gateways for first-time aesthetic toxin users, emphasizing experience, ambiance, and package-based offerings, while often relying on supervising physicians for clinical governance. Research and academic institutes, finally, act as engines of innovation, testing new protocols, indications, and modes of administration that may later diffuse into mainstream practice.
When viewed together, these segmentation layers reveal a market that is far from homogeneous. Strategic success depends on aligning product characteristics and service models with the precise mix of toxin type, formulation, administration technique, distribution pathway, application area, and end user setting being targeted. Companies that tailor their approach to these nuanced segments can better allocate investment, refine messaging, and build resilient positions across both aesthetic and medical domains.
Regional perspectives highlight how policy, culture, and healthcare systems shape botulinum toxin adoption worldwide
Regional dynamics strongly influence how the botulinum toxins market develops, as regulatory frameworks, cultural attitudes toward aesthetics, healthcare infrastructures, and income levels vary widely. In the Americas, the United States functions as a bellwether, combining high disposable income, a mature aesthetic industry, and well-established therapeutic use in neurology, rehabilitation, and pain management. Strong brand recognition and a dense network of dermatology clinics and medical spas drive high procedure volumes in facial aesthetics and non-surgical rejuvenation. At the same time, hospitals and specialized clinics across North and South America are progressively integrating toxin-based therapies into standard care pathways for muscle spasms and paralysis-related conditions, chronic migraine, and certain gastrointestinal disorders.Canada and Latin American countries, including Brazil and Mexico, contribute distinct growth patterns. In Canada, a publicly funded healthcare system and stringent regulation shape medical applications, while the private sector supports robust aesthetic demand. Brazil and Mexico have vibrant aesthetic cultures and high receptivity to minimally invasive procedures, often paired with price sensitivity and diverse provider quality. These markets reward companies able to balance premium positioning with accessible offerings and strong training to support safe practice. Across the Americas, digital marketing, social media influence, and medical tourism further amplify cross-border flows of patients, expertise, and brands.
Europe, the Middle East, and Africa present a heterogeneous but increasingly important set of opportunities. Western European countries typically enforce rigorous regulatory standards and demonstrate high awareness of both aesthetic and therapeutic indications. Systems of reimbursement and clinical guidelines are well developed, particularly for neurological and musculoskeletal uses, which encourages structured integration of toxins into long-term management plans. Central and Eastern European markets, while more cost-conscious, are expanding their aesthetic procedure bases and enhancing access to specialized care, often leveraging international training and partnerships.
In the Middle East, an expanding affluent population and strong emphasis on appearance support rapid uptake of facial aesthetics and non-surgical facelifts, especially in hubs such as the Gulf states. Private sector clinics and medical spas lead in adopting new techniques and premium brands, while governments in some countries are simultaneously investing in advanced neurology and rehabilitation services where medical toxin applications can flourish. Across Africa, the picture is more uneven, with select urban centers developing niche aesthetic and neurological treatment capabilities while large portions of the population remain underserved. Regulatory capacity, pricing, and infrastructure constraints shape opportunity, rewarding long-term, partnership-oriented strategies.
Asia-Pacific stands out for its demographic scale, rising middle class, and fast-evolving aesthetic norms. Countries like South Korea and Japan are trendsetters in minimally invasive beauty procedures, with sophisticated patient expectations and providers known for technical excellence. These markets emphasize natural, subtle enhancements and often integrate toxins into multi-modality regimens alongside fillers, lasers, and skincare. In China, rapid urbanization, increasing incomes, and expanding private healthcare drive strong interest in both facial aesthetics and medical uses, while authorities tighten oversight of product quality and supply chains.
Other Asia-Pacific markets, including India, Australia, and Southeast Asian countries, add to the region’s complexity. India combines a large population with growing awareness of aesthetic and therapeutic toxin applications, though insurance coverage and out-of-pocket costs significantly influence uptake. Australia features a mature aesthetic segment and advanced clinical practices in neurology and pain management, while Southeast Asia hosts emerging hubs where medical tourism, particularly for cosmetic procedures, is gaining momentum. Collectively, Asia-Pacific requires finely tuned strategies that factor in regulatory diversity, varied cultural expectations about beauty and aging, and differences in healthcare financing.
Across all regions, cross-border information flows and traveling patients are accelerating the diffusion of new techniques and brands. However, local reimbursement policies, training infrastructure, and enforcement against counterfeit or unauthorized toxins remain critical determinants of sustainable growth. Regional insight, therefore, is not merely a question of mapping demand but of understanding how systems, cultures, and policies interact to shape the real-world use of botulinum toxins.
Competitive intelligence shows leading and challenger companies racing to pair high-quality toxins with integrated service ecosystems
The competitive environment in the botulinum toxins market is characterized by a small number of globally recognized brands alongside an expanding group of regional players and innovators. Established manufacturers have built their positions through long-term clinical development, extensive safety data, and consistent quality control over complex biological production processes. Their products often enjoy strong brand loyalty among dermatologists, neurologists, and plastic surgeons, who rely on predictable performance and robust training and support programs.These leading companies increasingly differentiate themselves not solely on the intrinsic characteristics of their toxin formulations but also on the breadth of their service ecosystems. Educational initiatives, including hands-on workshops, digital learning platforms, and fellowship programs, play a central role in cementing relationships with providers. Many also offer practice development resources, such as guidance on patient communication, treatment planning, and clinic workflow optimization, particularly in the aesthetic arena where patient experience and retention are paramount.
Challenger firms and regional competitors are gaining ground by addressing unmet needs in pricing, formulation, and access. Some focus on developing cost-competitive alternatives that meet stringent regulatory standards, targeting markets and segments where affordability is a critical barrier. Others invest in novel delivery systems, such as extended-duration toxins or formulations tailored to specific indications, aiming to carve out differentiated niches. In markets with evolving regulatory maturity, these players must navigate varying levels of oversight and work to build trust with clinicians who may be cautious about adopting newer brands.
A growing emphasis on evidence generation and real-world data is further shaping company strategies. Regulators, payers, and healthcare providers are calling for more information on long-term safety, comparative effectiveness, and patient-reported outcomes, particularly for chronic medical uses such as muscle spasticity, pain management, and gastrointestinal disorders. As a result, companies are investing in post-marketing studies, registries, and data analytics capabilities to demonstrate the value of their products in diverse clinical settings. Those that can convert real-world insights into improved labeling, better educational content, and refined dosing guidance gain a competitive edge.
Digital engagement has become another crucial frontier. Companies are leveraging online platforms to provide treatment planning tools, injection technique videos, and peer-to-peer forums for clinicians. In the aesthetic segment, some are experimenting with consumer-facing initiatives to improve awareness, combat misinformation, and support safe decision-making. This requires careful balance to comply with varying promotional regulations across regions, but it also offers a powerful channel for reinforcing brand narratives and aligning perceptions with scientific evidence.
Partnerships and acquisitions are also shaping the competitive landscape. Larger pharmaceutical and medical aesthetics firms are acquiring or collaborating with smaller innovators developing complementary technologies, such as energy-based devices, imaging tools, or digital solutions that enhance treatment planning. These moves signal a shift from product-centric competition toward integrated offerings that combine toxins with other modalities and services. For industry participants, staying attuned to such collaborations and consolidation trends is essential to understanding how value chains and bargaining power are evolving.
In this environment, companies that maintain manufacturing excellence, invest in continuous medical education, harness real-world evidence, and build multi-modal, digitally enabled solutions are positioned to lead. Those that underinvest in quality systems, clinician support, or data transparency risk being sidelined as providers gravitate toward partners that help them navigate both clinical complexity and rising patient expectations.
Actionable strategies to strengthen positioning, resilience, and clinical relevance in the evolving botulinum toxins market
Industry leaders operating in the botulinum toxins space face an environment rich with opportunity but also marked by tightening regulatory expectations, evolving trade policies, and intensifying competition. To thrive, organizations should prioritize a set of actionable strategies that align with both near-term challenges and long-term shifts in clinical practice and consumer behavior.A central recommendation is to deepen segmentation-based strategy. Rather than treating the market as a monolith, companies should refine offerings and messaging for distinct combinations of toxin type, formulation, administration mode, and application. For example, high-volume aesthetic practices may prioritize ready-to-use liquid formulations and support for facial anatomy mastery, while hospitals treating complex muscle spasms and paralysis cases may require comprehensive dosing algorithms, multidisciplinary training materials, and strong pharmacovigilance support. By tailoring value propositions to each end user segment, leaders can enhance product relevance and strengthen loyalty.
Another imperative is to build resilience into supply chains and pricing models in anticipation of policy shifts such as United States tariffs planned for 2025. Firms should evaluate their exposure to cross-border cost volatility, identify critical materials and manufacturing steps at risk, and develop contingency plans, including diversifying suppliers or expanding regional production capacity where feasible. Transparent communication with providers about supply reliability and any necessary pricing adjustments will be crucial to maintaining confidence and minimizing disruptions to patient care.
Investing in real-world evidence and outcomes-based narratives should also be a priority. As payers and healthcare systems push for demonstrable value, companies that can document long-term safety, functional improvement, and quality-of-life gains in indications such as chronic migraine, spasticity, and pain management will be better placed in reimbursement discussions. In the aesthetic domain, structured data on satisfaction, duration of effect under different protocols, and retreatment patterns can differentiate offerings in crowded markets. Integrating these insights into educational materials and decision-support tools enhances both clinical practice and brand equity.
Digital capabilities need to move from peripheral initiatives to core differentiators. Leaders should develop integrated digital strategies that support clinicians throughout the treatment lifecycle, from training and planning to documentation and follow-up. Tools that assist with injection mapping, dosing estimation, and adverse event monitoring can elevate provider confidence and consistency. Parallel consumer-facing resources, carefully aligned with regulatory requirements, can help set realistic expectations, encourage adherence to follow-up schedules, and steer patients toward qualified providers.
Clinical education remains a non-negotiable area for investment. As new formulations, advanced techniques such as non-surgical facelifts, and emerging therapeutic indications proliferate, there is a growing need for high-quality training across dermatology clinics, hospitals, and medical spas. Industry leaders should expand mentorship networks, offer tiered education programs tailored to different experience levels, and support multidisciplinary forums where clinicians from neurology, gastroenterology, pain medicine, and aesthetics can share insights. Such initiatives not only raise standards of care but also embed products into practice patterns.
Finally, strategic partnerships can unlock new avenues of growth. Collaborations with device manufacturers, digital health firms, and academic research centers can accelerate innovation in combined therapies, novel indications, and patient engagement models. Leaders should actively scan for partnership opportunities that fill capability gaps, enable entry into new segments or geographies, or provide access to cutting-edge techniques and technologies. By adopting a partnership mindset, companies can move beyond incremental product improvements toward creating integrated solutions that address the full spectrum of clinical and patient needs.
Collectively, these recommendations point toward a more sophisticated, ecosystem-oriented approach. Organizations that execute on segmentation, resilience, evidence generation, digital transformation, education, and partnership will be best positioned to shape the future of the botulinum toxins market rather than merely respond to its changes.
Robust, multi-source research methodology providing a nuanced and validated view of the botulinum toxins marketplace
A rigorous research methodology underpins a robust understanding of the botulinum toxins market, ensuring that strategic insights rest on transparent and credible foundations. The analytical framework integrates both qualitative and quantitative dimensions, combining structured data collection with expert interpretation to capture the full complexity of this biologics-driven segment.Primary research serves as a cornerstone of this approach. In-depth interviews and discussions with dermatologists, plastic surgeons, neurologists, pain specialists, gastroenterologists, and rehabilitation physicians provide direct insight into prescribing behavior, treatment protocols, and evolving perceptions of different toxin types and formulations. Conversations with practice managers, medical spa owners, and hospital procurement teams add another layer of understanding around operational constraints, pricing sensitivity, and the practical realities of integrating toxins into clinical and aesthetic service lines.
These insights are complemented by engagement with regulatory and policy experts who clarify evolving rules around biologic manufacturing, pharmacovigilance, and promotional practices across key regions, including the Americas, Europe, the Middle East, Africa, and Asia-Pacific. Such perspectives are especially valuable for understanding how national and regional authorities approach issues like product registration, safety monitoring, and enforcement against counterfeit or unauthorized products.
Secondary research provides the structural context necessary to interpret primary findings. Analysis of publicly available regulatory documents, clinical guidelines, scien
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China Botulinum Toxins Market
Companies Mentioned
The key companies profiled in this Botulinum Toxins market report include:- AbbVie Inc.
- Ajinomoto Bio-Pharma Services
- ATGC Co.,Ltd.
- Bioplus Co., Ltd.
- BMI KOREA CO., LTD.
- BNC KOREA, INC.
- Chong Kun Dang Pharmaceutical Corp.
- Croma-Pharma GmbH
- Daewoong Pharmaceuticals Co.Ltd.
- Eirion Therapeutics, Inc.
- Evolus, Inc.
- Galderma SA
- Genetox Co., Ltd.
- Gufic Biosciences Ltd.
- HUGEL, Inc.
- Hugh Source International Ltd.
- Huons Global Co., Ltd.
- INIBIO Co., Ltd.
- Ipsen Pharma
- JETEMA, Co., Ltd.
- Lanzhou Institute of Biological Products Co. Ltd.
- Medytox Co., Ltd.
- Merz Pharma GmbH & Co.KGaA
- Object Pharma, Inc.
- PharmaResearch Co. Ltd
- Revance Therapeutics, Inc.
- Shanghai Fosun Pharmaceutical (Group) Co., Ltd.
- Supernus Pharmaceuticals, Inc.
- Taj Life Sciences Pvt. Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | January 2026 |
| Forecast Period | 2025 - 2032 |
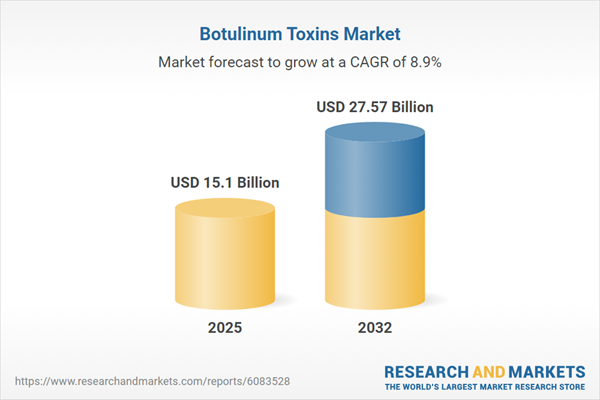
| Estimated Market Value ( USD | $ 15.1 Billion |
| Forecasted Market Value ( USD | $ 27.57 Billion |
| Compound Annual Growth Rate | 8.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |