Global Centesis Catheters Market Overview
Centesis catheters are used to drain out the excess fluids from the pleural cavities on the body or abdomen which sometimes could be infected. The drainage of these fluids results in reduced pressure on the organs which may cause other severe conditions if left untreated.The increasing technological advancements in the development of catheters are driving the global centesis catheters market demand. The increasing preference for minimally invasive surgical procedures is also driving the market growth as the catheters are inserted by making small incisions on the targeted area of the body. The market is further expected to be driven by the launch of new innovative developments by key players. Shockwave Medical, a cardiovascular medical device company based in Santa-Clara, California, announced full commercial availability for its newly developed C2+ coronary intravascular lithotripsy (IVL) catheter which can be used in severely calcified coronary artery disease.
Rising Strategic Acquisitions
In April 2023, Shockwave Medical completed its acquisition of Neovasc for USD 147 million. A sum of USD 27.25 per share was paid to Nevosac by Shockwave Medical. This deal acquired an enterprise value of nearly USD 100 million . Such acquisitions have the potential to influence the global centesis catheters market growth positively. Following this acquisition, Shockwave is expected to gain access to Neovasc's technologies, expertise, and intellectual property related to catheters.The market is expected to experience competition and expansion owing to such acquisitions. Acquisitions like these are major factors responsible for increased research and development activities in the market. The increased research and development activities are directly related to increased investment by the key players to stay ahead in the market.
Additionally, in October 2023, leading diagnostic and therapeutic medical technology company, Laborie Medical Technologies Corp. (Laborie), acquired Urotronic, Inc. (Urotronic), a private medical device company that developed Optilume® drug-coated balloon technology used in interventional urology to treat urethral strictures and benign prostatic hyperplasia (BPH) or enlarged prostate conditions.
This acquisition has the potential to bring more advanced and more effective solutions in interventional urology. This could also result in improved treatment options among patients suffering from urethral strictures and benign prostatic hyperplasia (BPH) or enlarged prostate conditions.
FDA Approvals to Aid the Centesis Catheters Market Growth
The market growth is also driven by the FDA approvals resulting in the launch of new products in the market. In May 2023, Abbott announced FDA approval of its TactiFlex Ablation Catheter. The company claims this product to be sensor-enabled, which is the world's first ablation catheter with a flexible tip and contact force technology. The TactiFlex catheter can be used in conjunction with Abbott's EnSite X EP System to provide physicians with a more precise visualization of the heart's anatomy. Abbott officials have stated that their next-gen radiofrequency ablation system will improve the efficiency and accuracy of arrhythmia treatments.Rise in Funds and Investments
In November 2023, Laborie Medical Technologies Corp., a leading diagnostic and therapeutic medical technology company, announced a strategic equity investment in Bright Uro, an early-stage medical technology company headquartered in Irvine, CA. Bright Uro is developing the Glean Urodynamic System™, a wireless, catheter-free, urodynamic monitoring system intended to quantify the pressure characteristics of the lower urinary tract. The catheter-free approach aligns with the increasing preference for minimally invasive and patient-friendly diagnostic technologies, more efficient and comfortable alternatives to address lower urinary tract issues, propelling the market growth.Global Centesis Catheters Market Segmentations
Centesis Catheters Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Type
- Small-bore Centesis Catheters
- Large-bore Centesis Catheters
Market Breakup by Procedure
- Paracentesis
- Thoracentesis
- Arthrocentesis
- Amniocentesis
- Others
Market Breakup by Applications
- Diagnostic Applications
- Therapeutic Application
- Palliative Care Applications
Market Breakup by End User
- Hospitals
- Ambulatory Surgical Centers
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Centesis Catheters Market Regional Analysis
The market has been witnessing significant growth due to the increased use of centesis catheters in several diseases. The increasing prevalence of chronic diseases such as cancer and lung-related lethal diseases is driving the market growth. To treat this condition, thoracentesis is performed to examine and treat the pleural effusions. To remove the excess fluids from the abdomen, paracentesis is used where a thin needle is inserted to relieve the pressure from the abdomen of the patient. The increasing popularity of image-guided centesis procedures among healthcare professionals to treat patients is also likely to contribute to the rising centesis catheters market share.In May 2023, Abbott (NYSE: ABT) announced that the U.S. Food and Drug Administration (FDA) has approved the company's TactiFlex™ Ablation Catheter, Sensor Enabled™, the world's first ablation catheter with a flexible tip and contact force technology. More than 37 million people worldwide live with AFib3, and it is expected to more than double by 2050. An additional five million cases are diagnosed every year, indicating a growing health challenge that demands innovative solutions for patients and their physicians.
The TactiFlex catheter is used to perform an ablation procedure to treat atrial fibrillation (AFib), the most common abnormal heart rhythm, and can result in reduced procedure times and better safety when compared to the company's previous generation catheters. This FDA approval enables the world's first ablation catheter with a flexible tip and contact force technology to fill a crucial gap of unmet needs in the medical sector, bolstering the global centesis catheters market demand.
Global Centesis Catheters Market: Competitor Landscape
In October 2023, a leader in smart infusion therapy, B. Braun Medical Inc. (B. Braun), announced the launch of its new Introcan Safety® 2 IV Catheter with Multi-Access Blood Control. The Introcan Safety 2 IV Catheter is the latest development from B. Braun in a long line of passive needlestick prevention catheters. In addition to automatic needlestick protection, clinicians are protected from blood exposure by a blood control feature every time the hub is accessed which reduces the risk of bloodborne pathogen exposure throughout the IV process.The key features of the centesis catheters market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:
- B. Braun Melsungen AG
- AngloDynamics
- Boston Scientific Corporation
- Becton, Dickinson and Company
- Cardinal Health
- Galt Medical Corp
- Medtronic Plc
- Merit Medical Systems
- Teleflex Incorporated
- Avanos Medical, Inc.
- Axiom Medical Consulting, LLC
- Medical Components, Inc.
- Mermaid Medical A/S
- Neuromedex GmbH
- Ningbo Honde Medical Instruments Co. Ltd.
- AngioDynamics, Inc.
- Argon Medical Devices, Inc.
- KM Medical
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- B. Braun Melsungen AG
- AngloDynamics
- Boston Scientific Corporation
- Becton, Dickinson and Company
- Cardinal Health
- Galt Medical Corp
- Medtronic Plc
- Merit Medical Systems
- Teleflex Incorporated
- Avanos Medical, Inc.
- Axiom Medical Consulting, LLC
- Medical Components, Inc.
- Mermaid Medical A/S
- Neuromedex GmbH
- Ningbo Honde Medical Instruments Co. Ltd.
- AngioDynamics, Inc.
- Argon Medical Devices, Inc.
- KM Medical
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
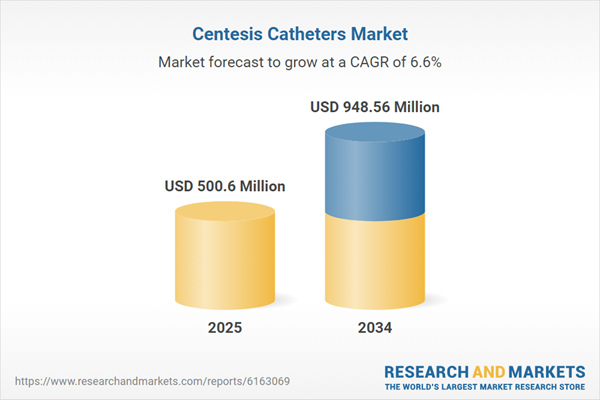
| Estimated Market Value ( USD | $ 500.6 Million |
| Forecasted Market Value ( USD | $ 948.56 Million |
| Compound Annual Growth Rate | 6.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 18 |