Speak directly to the analyst to clarify any post sales queries you may have.
An authoritative clinical and commercial introduction that explains device characteristics, stakeholder expectations, regulatory considerations, and the unmet needs driving implant adoption
The collagen meniscus implant is positioned at the intersection of orthopedics, regenerative medicine, and device innovation, responding to persistent clinical challenges in meniscal injury management. Clinicians increasingly seek biologically active scaffolds that can support tissue integration while maintaining mechanical stability. Consequently, device design priorities emphasize scaffold biocompatibility, predictable resorption profiles, and ease of implantation that align with existing arthroscopic workflows. In parallel, surgeons and procurement teams assess clinical evidence, procedural efficiency, and downstream patient outcomes as primary drivers of adoption.Stakeholders across clinical, regulatory, and payer domains are shaping the pathway to wider adoption. Clinicians prioritize robust clinical data demonstrating improved functionality and reduced progression to osteoarthritis. Regulatory authorities require clear safety and performance evidence that aligns with device classification and equivalence frameworks. Payers and hospital procurement groups increasingly evaluate value propositions in the context of procedural cost, long-term outcomes, and readmission risk. Taken together, these forces are defining both near-term commercialization imperatives and longer-term innovation roadmaps for implant developers.
Transformative shifts in biomaterials, surgical technique, and evidence expectations that are reshaping how collagen meniscus implants are developed, used, and commercialized
The landscape for collagen meniscus implants is undergoing rapid transformation driven by advances in biomaterials science, surgical technique, and evidence generation. New scaffold architectures and crosslinking chemistries have enhanced tissue integration and mechanical resilience, enabling implants to better mimic native meniscal mechanics. Concurrently, integration of biologics and adjunctive intraoperative therapies has shifted clinician expectations toward combined solutions that support biological repair rather than simple mechanical replacement. These technical shifts are complemented by improvements in arthroscopic instrumentation and imaging, which reduce procedural complexity and broaden the range of cases considered amenable to implant-based intervention.Market dynamics are also evolving due to changes in procurement practices, a growing emphasis on real-world evidence, and rising expectations for long-term functional outcomes. Health systems are increasingly focused on total cost of care and patient-reported outcomes, prompting device developers to prioritize evidence demonstrating reductions in downstream interventions and improved quality of life. Moreover, strategic collaborations between device companies, academic centers, and contract manufacturers are accelerating development cycles while creating more diverse commercialization pathways. As a result, competitive differentiation increasingly rests on a combination of clinical data, operational compatibility, and integrated services that support adoption at scale.
How recent tariff shifts and evolving trade dynamics in the United States are creating supply chain, cost, and procurement implications for orthopedic implant stakeholders
Trade policy developments in 2025 have intensified scrutiny of supply chains and cost structures within the orthopedics sector, with tangible implications for collagen meniscus implant stakeholders. Many raw materials and specialized components are sourced across borders, so changes in import duties or customs procedures increase the cost and lead-time variability for manufacturers. These pressures have prompted some firms to reassess supplier portfolios, accelerate qualification of alternative suppliers, and consider nearshoring or regional manufacturing hubs to mitigate exposure to trade-related disruptions. In turn, procurement teams within hospitals and surgical centers are adapting sourcing strategies to prioritize supply resilience alongside traditional criteria such as price and clinical performance.The cumulative effect of tariff-related adjustments extends beyond unit cost considerations to influence capital allocation and commercialization timelines. Increased input costs can compress margins or require price adjustments, which may alter purchasing dynamics within sensitive reimbursement environments. For device developers, this environment underscores the importance of transparent cost models, flexible manufacturing footprints, and proactive engagement with payers to articulate value that offsets short-term cost pressures. Additionally, manufacturers with diversified supplier networks and localized production capabilities are better positioned to maintain continuity of supply and support long-term partnerships with health systems.
Strategic segmentation insights that integrate end-user contexts, clinical applications, distribution pathways, and tear-type considerations to inform product and go-to-market decisions
A granular view across end users, applications, distribution channels, and tear types reveals differentiated adoption pathways and commercialization priorities. From an end-user perspective, ambulatory surgical centers encompass hospital-based centers and standalone centers and tend to prioritize efficiency, streamlined implants that shorten operative time and fit into high-throughput workflows. Hospitals include private hospitals and public hospitals and often require robust clinical evidence and integration with broader care pathways, while orthopedic clinics composed of hospital-based clinics and standalone clinics emphasize outpatient suitability and cost-effectiveness. Application-driven segmentation differentiates clinical usage scenarios such as meniscal repair, meniscal replacement, and partial meniscectomy, each demanding distinct device features and supporting evidence. Devices designed for repair must facilitate biological healing and fixation, whereas replacement options emphasize load-bearing durability and anatomical conformity; partial meniscectomy adjuncts require simplicity and low procedural overhead.Distribution channels also shape commercialization tactics and go-to-market investments. Direct tender environments split into government tenders and private tenders and favor suppliers that can demonstrate compliance, competitive pricing, and reliable service. Distributor models, including national distributors and regional distributors, offer breadth of reach and logistical support but require tailored commercial programs and training. Online channels such as e-commerce platforms and manufacturer websites are emerging as complementary paths for informational engagement and certain direct-to-clinic transactions. Finally, tear-type segmentation across complex tears, degenerative tears, and traumatic tears influences clinical messaging, patient selection criteria, and outcomes measurement. Complex tears often necessitate devices with enhanced fixation and biological augmentation, degenerative tears focus attention on symptomatic relief and preservation of joint health, and traumatic tears emphasize timely intervention and restoration of mechanical function. Together, these segmentation lenses inform product design, evidence strategies, training programs, and distribution models that align with distinctive clinical and procurement requirements.
Key regional perspectives on regulatory complexity, reimbursement variability, and localized commercialization strategies across major global geographies
Regional dynamics influence regulatory pathways, reimbursement structures, clinical practice patterns, and supply chain strategies in distinct ways. In the Americas, regulatory oversight and reimbursement mechanisms shape adoption through established pathways for device approval and hospital procurement practices that often emphasize clinical evidence and cost-effectiveness. Market participants operating in the Americas must address diverse payer environments and regional variations in hospital procurement governance while leveraging centers of excellence to build clinical momentum. In Europe, Middle East & Africa, regulatory frameworks and reimbursement policies vary widely; this region requires nuanced market access plans that account for national-level regulatory requirements, varied tendering practices, and differing levels of infrastructure to support advanced arthroscopic procedures. Local clinical champions and region-specific evidence can accelerate uptake where reimbursement aligns with demonstrated improvements in function and reduced downstream interventions.Asia-Pacific presents a heterogeneous landscape characterized by rapid adoption in some high-income jurisdictions and distinct reimbursement and manufacturing dynamics in emerging markets. Local manufacturing presence and partnerships with regional distributors can be decisive for market access in Asia-Pacific, especially where import pathways are constrained or procurement favors domestic suppliers. Across all regions, demographic trends such as aging populations and the prevalence of sports-related injuries drive clinical demand, while digital health adoption and telemedicine expand the channels for patient follow-up and outcomes tracking. Successful regional strategies therefore blend regulatory agility, payer engagement, localized distribution, and evidence generation attuned to the specific clinical and procurement realities of each geography.
Company-level competitive analysis focused on clinical evidence, manufacturing resilience, distribution strategies, and integrated service models that drive adoption
Competitive dynamics among companies in this space are shaped by clinical evidence generation, manufacturing capabilities, intellectual property, and the capacity to support integrated service delivery. Leading firms differentiate through robust clinical programs that demonstrate safety, durability, and patient-centered outcomes, while newer entrants focus on niche material innovations or streamlined surgical workflows that address specific clinical gaps. Strategic partnerships between device developers and academic or clinical centers accelerate evidence generation and can create referral pathways that support market entry. Manufacturing strength, including validated supply chains and scalable production processes, is increasingly a competitive advantage given the need for resilient supply in the face of trade and logistics pressures.Commercially, successful companies combine targeted clinician education, strong KOL networks, and tailored reimbursement support to reduce barriers to adoption. Distribution strategies that integrate direct tender engagement with national and regional distributor partnerships can expand reach while maintaining service quality. Additionally, after-sales services such as surgeon training, procedure support, and outcomes monitoring are becoming integral to the value proposition, enabling firms to demonstrate longitudinal impact and strengthen customer relationships. Intellectual property and differentiated device features remain important, but the most durable competitive positions will likely emerge from combinations of clinical credibility, operational reliability, and a demonstrated capacity to support system-level value creation.
Actionable strategic priorities for companies that balance clinical evidence, supply chain resilience, tailored commercialization, and payer engagement to accelerate meaningful adoption
Industry leaders should prioritize a multi-dimensional strategy that balances clinical validation, supply chain resilience, and pragmatic commercialization. First, accelerate clinical evidence generation through pragmatic trials and registry participation that capture functional outcomes and patient-reported metrics, thereby addressing the evidence needs of clinicians and payers. Concurrently, invest in supply chain redundancy and supplier qualification to mitigate exposure to cross-border trade disruptions and to ensure timely fulfillment for hospitals and outpatient centers. These investments support reliable market access and reduce the risk that procurement decisions will be driven solely by short-term cost considerations.Second, tailor commercialization programs to the distinct needs of end users and distribution channels by developing training curricula for hospital-based and standalone surgical centers, aligning product configurations with application-specific requirements such as repair versus replacement, and establishing partnerships with national and regional distributors to optimize reach. Engage early with payers and procurement groups to clarify value propositions and reimbursement pathways, and leverage digital tools for remote training and outcomes tracking to demonstrate real-world impact. Finally, consider strategic manufacturing and regionalization options that enhance responsiveness to local demand and regulatory requirements, while maintaining a clear roadmap for incremental product improvements informed by clinician feedback.
A rigorous mixed-method research approach combining expert interviews, regulatory and clinical evidence review, supply chain mapping, and stakeholder validation for robust insights
This research synthesizes primary qualitative interviews, secondary literature and regulatory review, and triangulation of supply chain and commercial intelligence to produce a comprehensive view of the landscape. Primary research included in-depth interviews with practicing orthopedic surgeons, hospital procurement leaders, ambulatory surgical center administrators, distribution partners, and device executives to capture practical insights on clinical decision-making, procurement drivers, and operational constraints. Secondary sources comprised peer-reviewed clinical literature, regulatory filings, and public domain clinical registries to validate device performance characteristics and safety profiles. Together, these inputs provided the evidence base for thematic analysis and hypothesis testing.Analytical methods included mapping of end-user workflows and procurement pathways, segmentation analyses that aligned clinical applications with distribution and facility types, and scenario-based assessment of supply chain vulnerabilities. Findings were validated through expert review sessions and cross-checked against publicly available regulatory and clinical records. This mixed-method approach balances qualitative depth with rigorous validation, enabling robust interpretation of trends and implications while identifying areas where further targeted primary research would deepen understanding.
A concise conclusion underscoring the need for integrated strategies that align clinical evidence, manufacturing agility, and market access to realize sustained impact
The evolution of collagen meniscus implants reflects a confluence of scientific progress, shifting clinical expectations, and changing commercial dynamics. Advances in biomaterials and surgical techniques are expanding the clinical use cases for biologically active implants, while procurement and reimbursement pressures are elevating the importance of demonstrable value and supply reliability. Consequently, successful commercialization requires more than a differentiated device; it demands an integrated strategy that aligns clinical evidence, manufacturing agility, and tailored market access programs.Looking ahead, stakeholders that invest in pragmatic evidence generation, supply chain diversification, and clinician education will be better positioned to navigate the complexities of adoption across diverse care settings. Collaboration across industry, clinical researchers, and payers will be essential to translate device-level innovation into measurable improvements in patient outcomes and system-level value. By focusing on these pillars, organizations can convert technological promise into sustained clinical impact and operational success.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Collagen Meniscus Implant Market
Companies Mentioned
The key companies profiled in this Collagen Meniscus Implant market report include:- Baxter International Inc.
- Bioventus Inc.
- CONMED Corporation
- Johnson & Johnson
- Medtronic plc
- Orthofix Medical Inc.
- Smith & Nephew plc
- Stryker Corporation
- Tissue Regenix Group plc
- Zimmer Biomet Holdings, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 188 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
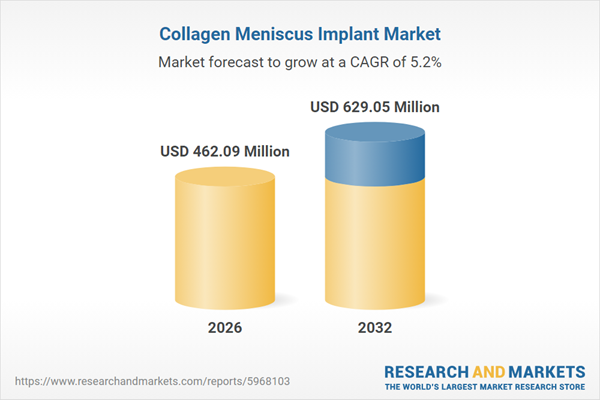
| Estimated Market Value ( USD | $ 462.09 Million |
| Forecasted Market Value ( USD | $ 629.05 Million |
| Compound Annual Growth Rate | 5.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |