Speak directly to the analyst to clarify any post sales queries you may have.
An authoritative orientation to how demographic, clinical, and technological forces are reshaping clinical practice and procurement choices for respiratory device stakeholders
The chronic respiratory disease landscape has entered an era of heightened clinical focus and commercial activity, driven by demographic shifts, evolving treatment paradigms, and rapid device innovation. As prevalence patterns change alongside increased awareness of chronic obstructive pulmonary disease and asthma, stakeholders across clinical, manufacturing, and distribution channels are re-evaluating how devices integrate with therapy regimens and care pathways. This introduction sets the stage by outlining the core drivers shaping device development, adoption, and procurement within care settings ranging from acute hospital environments to in-home care.Beyond epidemiology, the interplay between patient expectations, clinician preferences, and payer demands is reshaping requirements for usability, connectivity, and cost-effectiveness. Digital health enablers, such as adherence monitoring and remote patient management, are increasingly considered essential attributes rather than optional add-ons for new device introductions. Meanwhile, regulatory scrutiny and quality requirements demand tighter alignment between engineering design, human factors, and clinical evidence. This opening frames the discussion to follow, emphasizing how multi-stakeholder pressures converge to prioritize devices that deliver demonstrable clinical value, operational efficiencies, and seamless patient experiences.
How integrated digital ecosystems, supply chain resilience, and shifting care models are redefining competitive advantage and clinical adoption in respiratory device markets
The respiratory device landscape is undergoing transformative shifts that are changing competitive dynamics and patient pathways in fundamental ways. Device manufacturers are prioritizing integrated solutions that combine pharmacotherapy delivery with digital adherence tools and remote monitoring capabilities, reflecting a broader move from single-product offerings to ecosystem-based care propositions. This shift is prompting a re-evaluation of go-to-market models as companies seek partnerships with digital health firms, electronic health record integrators, and payers to demonstrate longitudinal outcomes and support value-based contracting.Concurrently, supply chain resilience and manufacturing localization are becoming strategic priorities. Manufacturers are diversifying sourcing strategies and investing in flexible production platforms that can scale across device types while meeting heightened regulatory documentation and quality expectations. Clinical practice is also evolving: care models increasingly emphasize outpatient management and home-based interventions, placing a premium on devices that are intuitive, portable, and reliable outside institutional settings. Taken together, these shifts are not incremental; they are reconfiguring product roadmaps, commercial alliances, and clinical adoption pathways in ways that will determine leadership positions over the next strategic cycle.
Understanding how trade policy adjustments can shift sourcing, manufacturing localization, and procurement behavior across the respiratory device ecosystem
The introduction of new tariff measures and policy shifts related to trade can create ripple effects that extend well beyond headline costs, influencing procurement strategies, manufacturing decisions, and pricing negotiations across the respiratory device ecosystem. Tariffs that increase the cost of imported components or finished devices encourage firms to reassess their supplier footprints and could accelerate near-shoring or the diversification of manufacturing sites. Such structural adaptation has implications for lead times, quality control protocols, and capital allocation, as manufacturers weigh the trade-offs between incremental cost reductions and the investment required to establish local production capability.For clinical and distribution stakeholders, tariffs can lead to changes in sourcing preferences and contracting behaviors. Health systems and group purchasing organizations may intensify efforts to secure long-term supply agreements or to favor vendors with local manufacturing capabilities to mitigate exposure to trade volatility. Distributors and pharmacies may be compelled to renegotiate margin structures, revise inventory practices, and adjust promotional strategies to maintain affordability for patients. Importantly, tariff-induced cost pressures often magnify the importance of differentiation on clinical performance, ease of use, and long-term total cost of care, prompting manufacturers to emphasize device features that deliver demonstrable economic offsets to end users and payers.
Segment-driven clarity on how device type, therapeutic indication, care setting, and distribution channels determine design priorities, adoption drivers, and commercial approaches
A nuanced segmentation view reveals important differences in adoption dynamics and strategic priorities across device categories, clinical indications, end-user settings, and distribution pathways. Based on Device Type, market analyses consider Dry Powder Inhaler, Metered Dose Inhaler, Nebulizer, Soft Mist Inhaler, and Spacer And Valved Holding Chamber, with Nebulizer technologies further differentiated into Jet Nebulizer, Mesh Nebulizer, and Ultrasonic Nebulizer; these distinctions matter because each subtype presents unique manufacturing requirements, user interfaces, and clinical suitability that influence clinician preference and patient adherence. The relative prominence of device types varies with therapeutic objectives and patient populations, meaning that device design priorities-such as dose consistency, portability, and cleaning protocols-are shaped by intended use scenarios.Based on Indication, the market is segmented into Asthma and COPD, and this split affects disease management strategies, device selection criteria, and clinical outcomes research priorities. The needs of younger asthma populations often emphasize portability, ease of use, and lifestyle integration, whereas COPD management frequently prioritizes delivery efficiency for patients with reduced inspiratory flow and may favor certain nebulizer modalities. Based on End User, devices are evaluated across Clinic, Home Care, and Hospital settings, each of which imposes different demands for durability, sterilization, training, and connectivity. Finally, Based on Distribution Channel, stakeholders operate through Hospital Pharmacy, Online Pharmacy, and Retail Pharmacy routes, with channel choice influencing inventory strategies, patient access programs, and after-sales support models. Integrating these segmentation lenses helps stakeholders prioritize investment in product features, training initiatives, and distribution partnerships aligned with real-world use cases.
How divergent regulatory regimes, payer dynamics, and manufacturing priorities across the Americas, Europe Middle East & Africa, and Asia-Pacific shape differentiated market entry and commercial optimization
Regional dynamics shape both the pace of innovation adoption and the commercial models most likely to succeed, with marked differences across the Americas, Europe, Middle East & Africa, and Asia-Pacific. In the Americas, market evolution is influenced by a combination of high healthcare spend, established payer systems, and growing emphasis on remote monitoring, which supports rapid uptake of devices that integrate digital adherence and telehealth features. Regulatory pathways and reimbursement frameworks in this region often reward demonstrated outcomes, creating opportunities for manufacturers that can align clinical evidence with robust post-market data collection.In Europe, Middle East & Africa, heterogeneity in regulatory regimes and healthcare funding models encourages differentiated market entry strategies, where local partnerships, adaptability to national reimbursement rules, and attention to affordability are paramount. Several markets in this combined region emphasize public procurement and tender-driven purchasing, which raises the importance of demonstrable cost-effectiveness and reliable supply chains. The Asia-Pacific region presents a distinct set of dynamics: rapid urbanization, expanding healthcare infrastructure, and a rising middle class are increasing device demand, while regulatory modernization and local manufacturing ambitions are encouraging investments in scalable production and region-specific designs. Recognizing these regional contrasts is essential for tailoring commercial strategies, prioritizing clinical evidence generation, and allocating manufacturing footprints to align with demand profiles and procurement norms.
Competitive dynamics driven by product-digital convergence, manufacturing modernization, and partnerships that accelerate evidence generation and clinical integration
Competitive behavior among device makers and allied technology firms underscores a marketplace where product differentiation and strategic partnerships are key determinants of success. Leading companies are broadening their portfolios to encompass both drug delivery mechanics and adjunctive digital services, focusing on human factors engineering, data interoperability, and lifecycle support to secure long-term relationships with health systems and patients. Smaller, specialized firms are concentrating on niche innovations-such as advanced mesh aerosolization or user-centric spacer designs-that can be licensed or bundled by larger manufacturers seeking rapid capability expansion.Collaboration between device manufacturers and digital health providers has intensified, producing bundled offerings that pair inhalation devices with adherence analytics, mobile coaching, and clinician dashboards. These alliances help manufacturers gather real-world evidence to support reimbursement dialogues and clinical adoption. At the same time, competition around manufacturing efficiency and supply continuity is prompting investments in automated assembly, modular production lines, and quality management systems that facilitate regulatory compliance across multiple jurisdictions. Overall, the competitive landscape favors organizations that can combine robust engineering, validated clinical outcomes, and scalable commercial models to meet the diversified needs of healthcare providers and patients.
Practical strategic priorities and implementation steps for device manufacturers to strengthen product value, supply resilience, and clinician adoption across care settings
Industry leaders should adopt a set of pragmatic actions to solidify competitive positions and to ensure devices meet the evolving expectations of clinicians, payers, and patients. First, prioritize investments in integrated product ecosystems that link delivery mechanics with adherence monitoring and clinical decision support, thereby strengthening value propositions for payers and care teams. Second, pursue flexible manufacturing strategies that combine near-shore capabilities with strategic backup suppliers to reduce exposure to trade disruptions and to enable faster responsiveness to demand shifts. Third, deepen clinician and patient engagement early in the design cycle through human factors testing and iterative pilots to optimize usability and reduce adoption friction in real-world contexts.Additionally, commercial leaders should align evidence generation plans with reimbursement requirements by integrating health economics measures into clinical studies and by leveraging real-world data to demonstrate long-term benefits. Sales and distribution strategies must be tailored to channel nuances, with differentiated approaches for hospital tenders, retail pharmacy networks, and online pharmacy platforms. Finally, cultivate strategic partnerships with digital health firms and health systems to co-develop post-market services and to unlock new contracting models tied to outcomes. These collective actions will enable organizations to navigate regulatory scrutiny, secure clinician trust, and deliver devices that materially improve patient outcomes while supporting sustainable commercial returns.
A rigorous mixed-methods research approach combining expert engagement, clinical and regulatory evidence review, and scenario analysis to underpin actionable, evidence-based insights
This study draws on a multi-method research approach that combines qualitative expert interviews, device performance literature, regulatory documentation review, and targeted stakeholder engagement to triangulate findings and ensure analytical rigor. Primary inputs include structured interviews with clinicians, procurement professionals, manufacturing experts, and distribution executives to capture frontline perspectives on device performance, usability, and procurement drivers. Secondary research encompasses peer-reviewed clinical studies, regulatory guidance documents, and public policy announcements to contextualize clinical and market dynamics while ensuring factual accuracy.To enhance reliability, evidence synthesis followed a standardized evaluation protocol that assessed the quality of clinical data, the applicability of human factors findings to distinct care settings, and the operational feasibility of manufacturing adaptations. The methodology also incorporated scenario analysis to evaluate the implications of supply chain disruptions and policy shifts on strategic choices, while explicitly avoiding speculative numeric forecasts. Throughout the research process, attention was given to transparency in source attribution and to alignment of analytical assumptions with accepted clinical practice and regulatory frameworks, supporting actionable conclusions for industry decision-makers.
Concise concluding synthesis highlighting the imperative for flexible platforms, evidence-aligned commercialization, and partnership-driven pathways to sustained adoption
In closing, the respiratory device arena is characterized by accelerating integration of digital functionality, heightened attention to supply chain resilience, and evolving care delivery models that privilege home and ambulatory settings. These trends create opportunities for companies that can deliver devices combining clinical reliability, usability, and data-driven care enablement. Stakeholders who align product development with clinician workflows, who invest in flexible production capacity, and who demonstrate economic value through outcomes evidence will be best positioned to capture sustained adoption across diverse markets.As the ecosystem continues to evolve, decision-makers should emphasize strategic flexibility: prioritize modular product platforms that can be adapted for specific indications and end-user settings; build partnerships that extend capabilities beyond hardware; and integrate post-market data collection into commercialization plans to support reimbursement discussions and continuous improvement. By doing so, manufacturers and channel partners will not only mitigate near-term operational risks but also create differentiated propositions that deliver measurable benefits to patients, providers, and payers.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China COPD & Asthma Devices Market
Companies Mentioned
- 3M Company
- AstraZeneca plc
- Boehringer Ingelheim GmbH
- Chiesi Farmaceutici S.p.A.
- Cipla Ltd.
- Fisher & Paykel Healthcare Corporation Limited
- GlaxoSmithKline plc
- Novartis International AG
- OMRON Corporation
- PARI Medical Holding GmbH
- ResMed Inc.
- Sandoz International GmbH
- Sanofi S.A.
- Teva Pharmaceutical Industries Ltd.
- Viatris Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 197 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
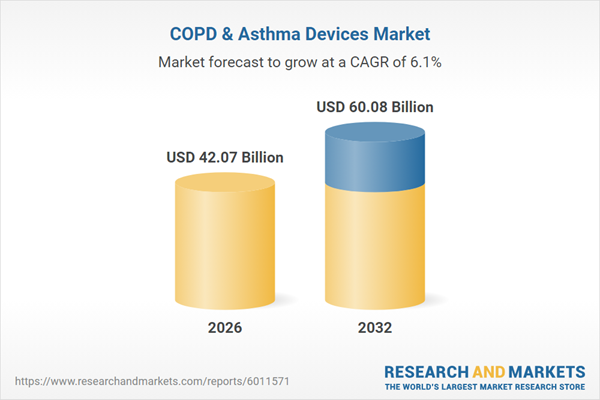
| Estimated Market Value ( USD | $ 42.07 Billion |
| Forecasted Market Value ( USD | $ 60.08 Billion |
| Compound Annual Growth Rate | 6.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |