Moreover, the developments in biotechnology, which include the discovery of targeted cytokine therapies and monoclonal antibodies, are driving market expansion. In addition, increased investments in research and development (R&D), as well as government efforts to promote innovative therapeutic approaches, are major factors augmenting the cytokine market share.
The market is driven by the growing incidences of autoimmune disorders, cancer, and inflammatory disorders that are inducing a greater need for cytokine therapeutic products. Moreover, the growth in research for immunotherapy is encouraging the development of new cytokine therapies, thereby increasing understanding of disease mechanisms and treatment effectiveness. For instance, on June 27, 2024, Foresite Capital launched a new fund focused on breakthrough technologies, including those in the biotech and technology sectors. Bright Peak Therapeutics raised USD 107 Million in Series B financing, led by RA Capital and supported by various investors.
The company is advancing its novel platform for synthesizing therapeutic cytokines, which enables enhanced cytokine biology and the creation of proprietary "Bright Peak Immunocytokines" by conjugating cytokines with specific antibodies. Apart from this, the regulatory support for biotechnology products is also accelerating the acceptance of cytokine therapies, which positively impacts the cytokine market outlook.
In the US, the market is driven by the increasing prevalence of chronic diseases, including cancer and rheumatoid arthritis, which are causing the demand for targeted therapies to increase. According to industry reports, arthritis impacts a large segment of the adult population in the United States, with more than 58 million individuals currently affected, half of whom are in their working years. By 2040, the number of adults in the country who have arthritis is anticipated to increase to 78 million. The availability of established healthcare infrastructure and a high level of healthcare expenditure also fuels the market.
Furthermore, a high level of investment in biopharmaceutical research, particularly in the fields of immuno-oncology and immunotherapy, has also led to an increase in cytokine development and research. Besides this, the changing regulatory landscape and the encouragement of clinical trials are also significant in propelling the approval of cytokine-based therapies.
Cytokine Market Trends:
Impact of Coronavirus Disease (COVID-19)The pandemic had a significant impact on the market, particularly through increased demand for cytokine-associated therapeutics. Throughout the pandemic, COVID-19 patients tended to present severe inflammatory reactions, such as cytokine storms, that led to complications and increased mortality. In June 2024, the World Health Organization (WHO) reported over 775 million cases and over seven million deaths globally since the onset of the pandemic. Consequently, therapies for cytokine-targeting to control such responses increased interest.
Moreover, the pandemic pushed forward the improvement and development of monoclonal antibodies and cytokine inhibitors, which were evaluated for their efficacy in controlling inflammation caused by COVID-19. Moreover, clinical trials for cytokine modulation in patients with COVID-19 give insights into cytokine-targeting interventions. This is opening up new research horizons for cytokine-based therapies, positioning cytokine therapeutics as crucial weapons in the fight against infectious diseases.
Increasing Prevalence of Cancer
The growing incidence of cancer across the globe is one of the key drivers of cytokine market growth. As per NCBI, by 2050, cancer cases worldwide are anticipated to reach 35.3 million, representing a 76.6% increase from the estimated 20 million in 2022. Likewise, cancer deaths are forecast to reach 18.5 million, showing an 89.7% growth compared to the 2022 estimate of 9.7 million. Cytokines play a crucial role in immune response and regulation, and their roles in tumor development and immune evasion have made them a central topic in cancer research. Immunotherapies, including those based on cytokines, are increasingly incorporated into the treatment of cancers resistant to conventional therapies.In addition to this, cytokine therapies are also studied in other types of cancers, such as melanoma, lung cancer, and renal cell carcinoma, where they seek to promote immune surveillance and anti-tumor activity. The increasing number of clinical trials and developments in cytokine-based therapies suggest that these drugs will be an integral part of cancer treatment in the future.
Expanding Alzheimer's Disease (AD) Research
Cytokine involvement in Alzheimer's Disease (AD) has gained significant attention due to the expanding research on neuroinflammation and its critical role in disease progression. The 2021 Global Burden of Disease (GBD) study estimated that approximately 129 million individuals across the globe lived with AD in 2021, and estimates suggest this will increase to 148 million by 2050. This rising prevalence reflects the escalating need for efficient therapeutic options. In Alzheimer's disease (AD), an inflammatory process in the brain, mediated by cytokines, is thought to play a role in neuronal injury and decline.Therefore, inhibition of pro-inflammatory cytokines, such as TNF-α and IL-1β, has emerged as a novel therapeutic strategy to reduce neuroinflammation and retard disease progression. Recent studies suggest that modulating these cytokines may provide new opportunities for treatment, thereby fueling interest in cytokine-based therapies. This has prompted pharmaceutical firms to invest in the discovery of new cytokine inhibitors and immunomodulators, and numerous clinical trials are currently ongoing.
Cytokine Industry Segmentation:
The research provides an analysis of the key trends in each segment of the global cytokine market, along with forecasts at the global, regional, and country levels from 2025-2033. The market has been categorized based on cytokine type, therapeutic application, and end user.Analysis by Cytokine Type:
- Tumor Necrosis Factor-TNF
- Interleukins-Il
- Interferons-IFN
- Epidermal Growth Factor-EGF
- Others
With the potential to target TNF, therapeutic opportunities have evolved, offering patients viable alternatives to traditional therapy. The rising prevalence of autoimmune diseases globally leads to growth in the application of TNF in the cytokine market. The expansion of the segment is further driven by continuing pharmaceutical innovation and a robust pipeline of new TNF inhibitors.
Analysis by Therapeutic Application:
- Cancer
- Asthma and Airway Inflammation
- Arthritis
- Others
The increasing prevalence of arthritis worldwide, along with the chronic nature of the disease, drives market expansion. The development of advanced treatments that are more efficient and have less negative impact is critical to expanding available therapeutic options for patients, ensuring that arthritis remains a key focus for cytokine-based treatment innovations. As a result, arthritis plays a major part in the market.
Analysis by End User:
- Pharmaceutical and Biotechnology Companies
- Contract Research Organizations
- Academic and Research Institutes
Their contribution is vital as these firms launch new cytokine-targeted therapies to market via clinical trials and approvals. The segment also forms partnerships, collaborations, and licensing deals, broadening their pipelines of products and facilitating the global supply of cytokine drugs. Pharmaceutical and biotech companies remain fundamental to the growth of the cytokine market as the need for personalized medicine and immunotherapies increases.
Contract Research Organizations (CROs) play a central role in the cytokine industry, aiding pharmaceutical and biotechnology firms with the research, development, and clinical evaluation of cytokine therapies. CROs offer critical services like clinical trial management, regulatory affairs, data analysis, and patient recruitment and assist in speeding up the development process of novel cytokine therapies.
Their knowledge enables organizations to effectively perform preclinical and clinical trials in the face of the intricate regulatory landscape. With the advancement in cytokine therapies, CROs provide assurance that treatments go through severe testing, safety evaluation, and efficacy checks. The growing demand for cytokine-based therapies necessitates CROs, which enable the timely and economic development of these life-saving therapeutics.
Academic and research institutions form the backbone of the market as they are leading centers of basic and applied studies in immunology, cell biology, and cytokine-related therapies. These institutions carry out cutting-edge research on the mechanisms of cytokines and how they influence disease advancement, thereby paving the way for innovative therapeutic strategies.
Several cytokine-based treatments available on the market today have stemmed from academic discoveries, which were subsequently developed into clinical uses by pharmaceutical companies. The institutes also partner with biotechnology companies and CROs, taking part in clinical trials and designing new treatment approaches. With increased emphasis on personalized and precision medicine, academic and research institutions will remain important drivers of future developments in the market.
Regional Analysis:
- North America
- United States
- Canada
- Asia-Pacific
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Others
- Europe
- Germany
- France
- United Kingdom
- Italy
- Spain
- Russia
- Others
- Latin America
- Brazil
- Mexico
- Others
- Middle East and Africa
North America also has regulatory systems that encourage fast-track approvals for new treatments, offering incentives for innovation, and creating a conducive environment for new cytokine therapies. With a heightened emphasis on precision medicine and immunotherapy, the region is still leading in cytokine-related clinical trials and drug development.
Key Regional Takeaways:
United States Cytokine Market Analysis
In 2024, the United States holds a substantial share of around 95.90% of the market share in North America. The market is primarily driven by the rising prevalence of autoimmune diseases, such as rheumatoid arthritis and Crohn's disease. As per the National Center for Biotechnology Information (NCBI), approximately 1.3 million American adults, representing 0.6-1% of the adult population, are affected by rheumatoid arthritis (RA). Among these patients, the prevalence of work-related disabilities associated with RA is estimated to be around 35%. In addition to this, the increasing incidence of cancer, driving the need for cytokine-targeted immunotherapies, is propelling the market growth.Similarly, continual technological advancements in cytokine biology, along with the development of novel biologic drugs, are enhancing treatment options, further augmenting market expansion. The growing focus on personalized medicine is also promoting the adoption of tailored cytokine therapies for individual patients. Furthermore, the expansion of clinical trials dedicated to cytokine-related treatments is accelerating the market's momentum. The ongoing shift towards biologic drugs over traditional therapies is another significant market driver. Besides this, the rise in the aging population, with its higher incidence of chronic diseases, is constantly pushing the market demand for cytokine therapies.
Europe Cytokine Market Analysis
The market for cytokines in Europe is experiencing growth driven by the rising incidence of chronic diseases, such as cardiovascular conditions and diabetes. According to WHO, diabetes is a prevalent chronic condition in the WHO European Region, which has the highest global burden of type 1 diabetes. Around 74 million adults and 300,000 children and adolescents are affected by diabetes in the region. By 2045, it is projected that 1 in 10 people in the region will have diabetes. In accordance with this, the increasing prevalence of inflammatory diseases like multiple sclerosis and psoriasis is further strengthening market demand.The continual advances in biotechnology, particularly in gene editing and CRISPR technology, are enabling the development of more effective cytokine therapies. Furthermore, the growing focus on personalized medicine, fueling the need for immune-modulatory treatments, is impelling the market. The advancement in research and development funding in immunotherapy and cytokine drug discovery is also propelling market innovation. Additionally, enhanced healthcare infrastructure and improved access to advanced therapies across Europe are contributing to market expansion. Moreover, strategic collaborations between biotech companies and research institutions, fostering the development of novel therapies, are providing an impetus to the market.
Asia Pacific Cytokine Market Analysis
The Asia Pacific market is predominantly propelled by the increasing prevalence of infectious diseases, such as hepatitis and HIV. Similarly, the rapid growth of the biotechnology and pharmaceutical sectors, particularly in China and India, is fueling market expansion. The rising adoption of advanced healthcare technologies, including precision medicine and gene therapy, is encouraging the integration of cytokine-based treatments. Furthermore, increasing government investments in healthcare infrastructure and biotechnology research are creating opportunities for market development.According to IBEF, the Indian government allocated INR 99,858 Crore (USD 11.50 Billion) to the healthcare sector in the Union Budget 2025-26, marking a 9.78% increase from the previous allocation of INR 90,958 Crore (USD 10.47 Billion) in FY25. The increased awareness among healthcare professionals about immunotherapies and cytokine-based treatments is supporting market growth. Moreover, the rising demand for treatments in cancer and autoimmune diseases is further driving the need for cytokine therapies in the region.
Latin America Cytokine Market Analysis
In Latin America, the market is advancing, driven by increasing healthcare investments in countries like Brazil and Mexico. As such, in September 2024, Rede D’Or, Brazil’s leading hospital group, planned to invest BRL 7.5 billion (USD 1.5 Billion) by 2028 to expand healthcare capacity. In line with this, the rising prevalence of chronic diseases such as diabetes and cancer is further contributing to growth in the market.Additionally, the expanding biotechnology sector, supported by government initiatives, is fostering innovation in cytokine treatments. Furthermore, the growing adoption of immunotherapy in cancer treatment is driving demand for cytokine-based therapies as healthcare providers seek more effective alternatives to traditional treatments to improve patient outcomes.
Middle East and Africa Cytokine Market Analysis
The market in the Middle East and Africa is significantly influenced by increasing government investments in healthcare, particularly in GCC countries. Furthermore, the rising incidence of autoimmune diseases and cancer is driving the demand for cytokine-based treatments, which is impelling market expansion. According to an industry report, the incidence of cancer in Saudi Arabia is rising significantly.Projections suggest a significant rise in new cases, from 27,885 in 2020 to an anticipated 60,429 by 2040, representing a staggering 116.7% rise. Additionally, growing collaborations between regional biotech companies and global pharmaceutical firms are fostering innovation in cytokine therapies. Besides this, continual advancements in healthcare infrastructure, alongside a focus on improving patient care, are facilitating the adoption of cytokine therapies across the region.
Competitive Landscape:
The market is dominated by a combination of mature pharmaceutical businesses and new biotech companies. These companies are engaged in the advancement and marketing of cytokine-based treatments aimed at diverse diseases, such as cancer, autoimmune diseases, and inflammatory diseases. The industry is very dynamic, with businesses specializing in novel therapeutic approaches, such as cytokine inhibitors, recombinant cytokines, and engineered cytokine-based treatments. Strategic partnerships, collaborations, and acquisitions are the norm as businesses strive to advance their product pipelines and gain competitive advantages in this evolving market.The cytokine market forecast projects that the market will witness tremendous growth in the coming years, fueled by developments in immunotherapy, the increased prevalence of chronic diseases, and heightened healthcare needs. In addition, one of the major trends propelling competition is the growing investment in research and development (R&D) to meet unmet medical needs, particularly for orphans and challenging diseases. Clinical trial success and regulatory approvals are key drivers determining the position in the market for players.
The report provides a comprehensive analysis of the competitive landscape in the cytokine market with detailed profiles of all major companies, including:
- AbbVie Inc.
- Abcam plc
- Amgen Inc.
- Applied Biological Materials Inc. (abm)
- Bio-Techne Corporation
- F. Hoffmann-La Roche AG
- GenScript Biotech Corporation
- Randox Laboratories Ltd.
- Thermo Fisher Scientific Inc.
- UCB S.A.
Key Questions Answered in This Report
- How big is the cytokine market?
- What is the future outlook of the cytokine market?
- What are the key factors driving the cytokine market?
- Which region accounts for the largest cytokine market?
- Which are the leading companies in the global cytokine market?
Table of Contents
Companies Mentioned
- AbbVie Inc.
- Abcam plc
- Amgen Inc.
- Applied Biological Materials Inc. (abm)
- Bio-Techne Corporation
- F. Hoffmann-La Roche AG
- GenScript Biotech Corporation
- Randox Laboratories Ltd.
- Thermo Fisher Scientific Inc.
- UCB S.A
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 138 |
| Published | June 2025 |
| Forecast Period | 2024 - 2033 |
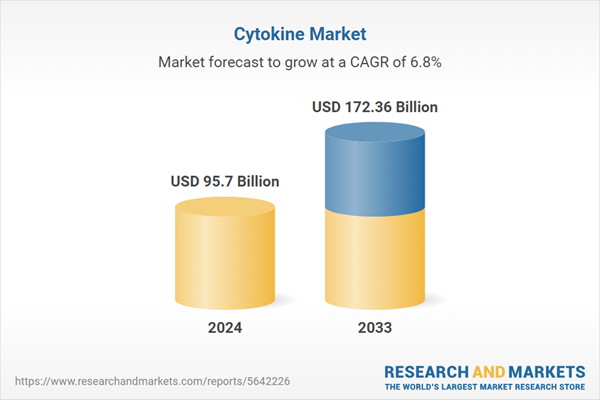
| Estimated Market Value ( USD | $ 95.7 Billion |
| Forecasted Market Value ( USD | $ 172.36 Billion |
| Compound Annual Growth Rate | 6.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |