Speak directly to the analyst to clarify any post sales queries you may have.
Concise strategic introduction to the evolving decongestant category driven by formulation innovation, regulatory scrutiny, and shifting patient and channel behaviors
The decongestant category occupies a unique space at the intersection of consumer self-care demand, prescription therapeutics, and rapidly evolving supply chain dynamics. Recent years have amplified the role of over-the-counter convenience and digital distribution while simultaneously raising regulatory scrutiny around active ingredients and age-appropriate dosing. Consequently, product developers, commercial strategists, and procurement teams must navigate a multi-faceted landscape where formulation choices, route of administration, and channel strategy collectively determine patient access and competitive positioning.Against this backdrop, the category’s core modalities-drops, nasal sprays, and tablets and capsules-continue to diversify in response to safety, convenience, and compliance considerations. Nasal delivery formats have seen innovation around metered dosing and single-use variants to address both efficacy and hygiene expectations, while oral solids and extended-release matrices aim to improve dosing adherence. At the same time, stakeholder priorities such as pediatric safety, geriatric tolerability, and adult convenience shape portfolio decisions. In short, understanding the interplay of consumer behavior, clinical guidance, and distribution mechanics is essential for evidence-based strategy formulation and resilient operational planning.
How technological innovation, regulatory tightening, and shifting consumer behavior are jointly transforming decongestant product development, labeling, and distribution
The landscape for decongestants is undergoing transformative shifts that are simultaneously technological, regulatory, and behavioral. On the technological front, precision dosing mechanisms and preservative-minimizing formulations have advanced, reducing adverse events and increasing consumer confidence. This trend is most evident in the evolution of nasal sprays from standard sprays to metered-dose formats and in the expansion of single-dose drop presentations that address hygiene and cross-contamination concerns. As a result, product differentiation increasingly hinges on device design and formulation compatibility rather than active ingredient alone.Regulatory evolution also exerts a pronounced influence. Authorities are intensifying scrutiny of pediatric labeling, age-specific dosing, and ingredient combinations, which compels manufacturers to invest in clinical substantiation and clearer labeling. Simultaneously, the digital transformation of healthcare and retail is reshaping distribution dynamics; online pharmacies and branded manufacturer channels are expanding access while enabling direct-to-consumer education and loyalty programs. These shifts interact with macro-level supply chain considerations, driving manufacturers to reassess sourcing strategies, contract manufacturing relationships, and packaging choices to maintain agility.
Consumer behavior further accelerates change. Adoption of self-care and demand for rapid symptomatic relief favors formats that are intuitive, portable, and perceived as safe. Consequently, oral and nasal modalities compete on convenience, onset of action, and perceived side-effect profiles. Taken together, these technological, regulatory, and behavioral forces are not isolated; they reinforce one another and produce an environment where agile product development, proactive regulatory engagement, and channel-specific commercialization will dictate competitive success.
Assessing the multifaceted cumulative consequences of United States 2025 tariffs on decongestant supply chains, sourcing strategies, and commercial pricing dynamics
The cumulative impact of United States tariffs announced for 2025 has reverberated across sourcing, pricing, and supply resilience considerations for decongestant producers and distributors. Many active pharmaceutical ingredients and specialized excipients used in decongestant formulations originate in global supply networks; tariffs on these inputs elevate landed costs and prompt procurement teams to reassess supplier portfolios. Consequently, some manufacturers accelerate qualification of alternative suppliers in tariff-exempt zones, while others examine reshoring or nearshoring options to mitigate long-term exposure.In addition, tariffs have influenced packaging and device sourcing decisions. Metered-dose components and single-use applicators often rely on precision manufacturing from specific geographies; increased import levies have made vertical integration or local tooling more attractive for companies seeking to stabilize unit economics. At the commercial interface, distributors and retail partners face margin compression, which in turn pressures pricing strategies and promotional investments. Many organizations respond by optimizing SKU assortments, prioritizing higher-margin formulations or device-enhanced offerings that justify price points through perceived clinical or convenience benefits.
Finally, tariffs have accelerated collaborative planning across the value chain. Manufacturers, contract manufacturers, and distributors increasingly use scenario planning and stress-testing to preserve product availability during tariff phase-ins. This has also prompted a renewed emphasis on regulatory and customs expertise to leverage preferential trade agreements where applicable. Overall, the tariff environment has not only raised short-term costs but also altered strategic priorities around supply chain localization, supplier diversification, and product portfolio rationalization.
Actionable segmentation insights reveal how product type, administration route, distribution channel, end-user groups, prescription status, and form factor determine competitive priorities
Segmentation reveals how therapeutic needs, user preferences, and channel mechanics converge to shape category performance and product development priorities. Based on product type, the category divides into drops, nasal sprays, and tablets and capsules, with drops further differentiated into multi dose and single dose; nasal sprays split between metered dose and standard spray; and tablets and capsules separated into extended release and immediate release formats. These distinctions matter because single-dose and metered-dose technologies often command a safety and hygiene premium, whereas extended-release oral matrices address adherence and dosing frequency.Based on route of administration, the category is studied across nasal and oral pathways, and this bifurcation influences both clinical performance and consumer choice. Nasal delivery tends to emphasize rapid onset and localized action, while oral approaches prioritize convenience and prolonged symptomatic control. Based on distribution channel, the category is observed across hospital pharmacies, online pharmacies, and retail pharmacies, with hospital pharmacies further differentiated into private and public institutions, online pharmacies split between branded websites and third-party platforms, and retail pharmacies distinguished as chain and independent operators. Channel segmentation informs go-to-market tactics and compliance obligations, as institutional procurement and e-commerce ecosystems demand different packaging, unitization, and information flows.
Based on end user, the market framework includes adults, geriatric populations, and pediatrics, with pediatrics further classified into adolescents, children, and infants; this granularity is essential for dosing, regulatory labeling, and safety communication. Based on prescription status, products are categorized as over the counter or prescription, influencing distribution control, marketing pathways, and professional engagement strategies. Finally, based on form, the product landscape spans liquid, semi-solid, and solid formats, with liquids subdivided into suspension and syrup, semi-solids into gel and ointment, and solids into capsule and tablet; each form factor carries distinct formulation constraints, stability requirements, and patient usability considerations. In sum, these layered segmentation lenses enable product teams to align formulation choices, messaging, and channel strategies with specific clinical and consumer needs.
Regional strategic imperatives across the Americas, Europe, Middle East & Africa, and Asia-Pacific that determine regulatory engagement, channel focus, and portfolio optimization
Regional dynamics materially influence regulatory frameworks, supply chain resilience, and consumer preferences in ways that demand region-specific strategies. In the Americas, pricing pressures, large retail banners, and a mature over-the-counter culture create opportunities for broad-reach promotional campaigns and rapid e-commerce scaling, while regulatory agencies maintain rigorous safety and labeling standards that favor well-documented formulations. Conversely, Europe, Middle East & Africa present a heterogeneous regulatory and access environment; this region combines advanced markets with stringent pharmacovigilance and emerging markets where affordability and distribution infrastructure drive different product choices and pack sizes.Asia-Pacific exhibits dynamic demand growth driven by urbanization, expanding retail pharmacy networks, and rising consumer health literacy, which in turn fosters adoption of device-enhanced nasal formats and branded online channels. However, Asia-Pacific also presents complex supply chain advantages, given its concentration of pharmaceutical ingredient manufacturing, which interacts with tariff and trade considerations. Across all regions, local reimbursement policies, national OTC frameworks, and cultural attitudes toward self-medication shape how manufacturers prioritize SKUs, trade marketing investments, and regulatory submissions. Therefore, successful regional strategies require nuanced alignment of product design, pricing, and channel engagement with distinct regional imperatives.
Key competitive intelligence on how multinational brands, regional specialists, contract manufacturers, and upstream suppliers shape product development and commercialization advantage
Competitive dynamics in the decongestant category reflect a mix of multinational pharmaceutical companies, national brand specialists, and contract manufacturers that supply both branded and private-label offerings. Large pharmaceutical players leverage scale and regulatory experience to introduce device-integrated formulations and extended-release oral matrices, while regional specialists often compete on speed to market, price competitiveness, and tailored formulation choices that reflect local clinical practice and consumer preferences. Contract manufacturing organizations play an outsized role in enabling smaller brands to access specialty dosing devices and complex liquid or semi-solid formulations without major capital investment.Partnerships between brand owners and logistics providers are increasingly common, particularly as online pharmacy channels require rapid fulfillment and returns management. Additionally, companies that invest in clinical and real-world evidence generation differentiate themselves by substantiating age-appropriate dosing and safety profiles, which is critical when engaging professional healthcare audiences and addressing pediatric labeling requirements. Lastly, upstream suppliers of active ingredients and device components wield strategic influence; their geographic footprint and pricing decisions directly affect product economics, particularly in environments affected by tariffs or supply constraints. Consequently, competitive advantage now flows from integrated capabilities across formulation science, device engineering, regulatory strategy, and channel execution.
Practical, high-impact strategic recommendations for leaders to align product innovation, sourcing resilience, and tailored channel strategies amid regulatory and tariff pressures
Industry leaders should pursue a set of pragmatic, high-impact actions that align product, channel, and supply priorities with evolving regulatory and trade contexts. First, prioritize product differentiation through device innovation and age-appropriate formulations that address hygiene, dosing precision, and adherence. Investing in metered-dose nasal sprays and single-dose drop technologies, while optimizing tablets for extended or immediate release where clinically relevant, creates clear value propositions for both consumers and professional prescribers. Moreover, ensure formulations are adapted for nasal and oral routes with clear labeling to reduce misuse and enhance patient confidence.Second, adopt a resilient sourcing strategy that balances supplier diversification with selective localization of critical components. Where tariffs and trade uncertainties increase cost volatility, nearshoring key inputs or qualifying multiple suppliers can preserve margins and prevent stock disruption. Third, refine channel strategies by tailoring SKUs and packaging to the needs of hospital pharmacies-both private and public-online pharmacies via branded websites and third-party platforms, and retail pharmacies whether chain or independent. Complement these moves with targeted professional education for clinicians and point-of-sale digital content for consumers. Finally, strengthen evidence generation around pediatric and geriatric use, engage early with regulators on labeling expectations, and use scenario planning to stress-test pricing and distribution strategies against tariff developments. Collectively, these recommendations enable leaders to protect revenue streams while pursuing growth via product and channel innovation.
Transparent research methodology combining primary stakeholder interviews, regulatory review, clinical literature synthesis, and scenario analysis to validate insights across segments and regions
This research synthesizes primary and secondary inputs to build a holistic understanding of the decongestant category across formulations, routes, channels, end users, and regions. The primary research component included structured interviews with senior commercial, regulatory, and supply chain stakeholders across manufacturers, distributors, hospital procurement units, and pharmacy groups, complemented by clinician interviews to validate safety and dosing priorities. Secondary research drew on public regulatory guidance, peer-reviewed clinical literature, product labeling databases, and supply chain trade publications to triangulate product attributes and distribution patterns.Analytical methods combined qualitative thematic analysis with scenario planning to assess the implications of trade policy shifts and channel evolution. Segmentation frameworks were applied to evaluate product-type differentiation-drops segmented into multi dose and single dose, nasal sprays split into metered dose and standard spray, and tablets and capsules categorized as extended release and immediate release-alongside route-of-administration considerations across nasal and oral pathways. Distribution channel analysis differentiated hospital pharmacies into private and public, online pharmacies into branded websites and third-party platforms, and retail pharmacies into chain and independent operators. End-user segmentation included adults, geriatric populations, and pediatrics with adolescents, children, and infants specified. Form analysis distinguished liquid into suspension and syrup, semi-solid into gel and ointment, and solid into capsule and tablet. Throughout, findings were validated through cross-referencing clinical guidance and stakeholder feedback to ensure actionable reliability.
Clear concluding synthesis highlighting how integrated product innovation, regulatory strategy, channel differentiation, and supply resilience will define success in decongestants
In conclusion, the decongestant category is at an inflection point where product innovation, regulatory clarity, channel modernization, and trade policy converge to reshape commercial opportunity. Product differentiation increasingly depends on device and formulation choices that address hygiene, dosing precision, and adherence across diverse end-user groups including adults, geriatric patients, and pediatric subgroups. At the same time, distribution strategies must reconcile the needs of hospital pharmacies-private and public-online pharmacies operating via branded websites and third-party platforms, and retail pharmacies in both chain and independent formats.Moreover, macro-level forces such as tariffs and supply-chain geopolitics have elevated the importance of sourcing resilience and supplier diversification. Companies that proactively align formulation investment with regulatory engagement, regional channel priorities across the Americas, Europe, Middle East & Africa, and Asia-Pacific, and scenario-based supply planning will be best positioned to sustain product availability and commercial momentum. Ultimately, success in this category will be determined by the ability to translate clinical credibility into compelling consumer propositions, operational resilience, and regionally tailored go-to-market execution.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China Decongestant Market
Companies Mentioned
The key companies profiled in this Decongestant market report include:- Alcon
- Aptar Pharma
- AstraZeneca PLC
- Aurobindo Pharma Limited
- Bausch Health Companies Inc.
- Bayer AG
- Boehringer Ingelheim International GmbH
- Church & Dwight Co., Inc.
- Cipla Ltd.
- Dr. Reddy's Laboratories Limited
- Eli Lilly and Company
- GlaxoSmithKline PLC
- Glenmark Pharmaceuticals Limited
- Haleon Group of Companies
- Johnson & Johnson Services, Inc.
- Perrigo Company PLC
- Procter & Gamble Company
- Reckitt Benckiser Group PLC
- Sanofi S.A.
- Sun Pharmaceutical Industries Limited
- Teva Pharmaceutical Industries Ltd.
- The Boots Company PLC
- Viatris, Inc.
- Walgreen Co.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 189 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
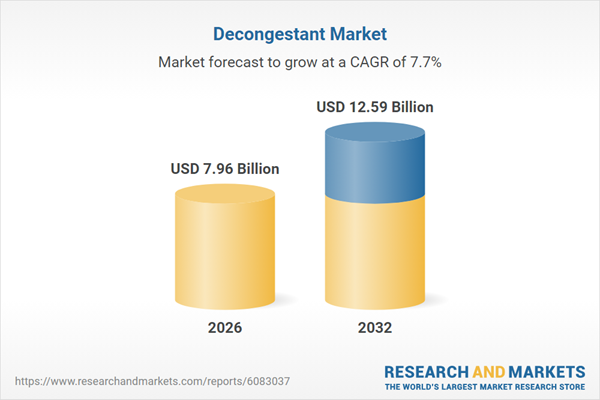
| Estimated Market Value ( USD | $ 7.96 Billion |
| Forecasted Market Value ( USD | $ 12.59 Billion |
| Compound Annual Growth Rate | 7.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |