Speak directly to the analyst to clarify any post sales queries you may have.
A concise synthesis explaining how recent dengue vaccine scientific advances intersect with operational realities to shape urgent strategic priorities for leaders
Dengue presents a complex and growing public health challenge in many tropical and subtropical regions, and developments in vaccine science have created new strategic imperatives for health systems, manufacturers, and investors. Recent advances in candidate platforms and regulatory approvals in multiple jurisdictions have shifted the conversation from proof of concept to operational readiness, where production capacity, cold chain logistics, and targeted immunization strategies determine real-world impact. Stakeholders now face a convergence of scientific progress and pragmatic constraints, including manufacturing scale-up, regulatory harmonization, and demand articulation across diverse healthcare settings.As vaccine candidates move through late-stage development and national immunization programs consider integration strategies, organizations must reconcile clinical evidence with implementation realities. This requires clarity on comparative safety and efficacy profiles across different vaccine types, dosage forms that influence programmatic suitability, and distribution pathways that determine access in urban and remote communities. In addition, demographic considerations such as age-specific safety and immunogenicity further complicate prioritization. Therefore, decision-makers need a concise, evidence-informed synthesis that connects clinical attributes to supply chain design and policy engagement.
This executive summary frames those connections by synthesizing recent technological breakthroughs, regulatory trends, and stakeholder motivations. It emphasizes practical actions that public health authorities, manufacturers, and commercial teams can deploy to translate scientific gains into broad, equitable protection against dengue. By focusing on operational levers and strategic trade-offs, the summary equips leaders to align clinical priorities with delivery capacity and long-term program sustainability.
An analysis of how technological diversification regulatory adaptation and delivery innovations are reshaping commercial and public health strategies for dengue vaccination
Over the past several years, the dengue vaccine landscape has experienced transformative shifts that realign priorities across research, manufacturing, and policy. Platform diversification has accelerated, with live attenuated and recombinant subunit technologies each demonstrating distinct development pathways and programmatic implications. As a result, resource allocation now emphasizes not only clinical differentiation but also manufacturability, cold chain efficiency, and dosing regimens that support rapid public health rollout. These shifts compel stakeholders to evaluate vaccines not solely on immunogenic endpoints but on end-to-end feasibility for sustained immunization efforts.Regulatory dynamics have similarly evolved, with health authorities increasingly favoring adaptive review processes and post-authorization evidence generation to balance timely access with patient safety. Consequently, manufacturers are investing in more robust pharmacovigilance systems and real-world evidence capabilities to support conditional approvals and incremental label expansions. Meanwhile, procurement models are changing as public purchasers and international agencies seek flexible contracting mechanisms that accommodate staged rollouts and risk-sharing arrangements. In response, commercial teams are adopting more collaborative engagement strategies with governments and NGOs to craft procurement terms that reflect unpredictable demand patterns.
Finally, system-level shifts in health delivery-driven by digital health tools, community-based vaccination platforms, and greater emphasis on equitable access-are reshaping distribution choices. The ability to deploy vaccines effectively in both urban centers and remote communities rests on aligning dosage form, cold chain considerations, and distribution channels with user preferences and infrastructure realities. Taken together, these transformative shifts underscore the importance of integrated planning that bridges clinical promise with logistical execution.
A strategic review of how 2025 tariff realignments in the United States accelerated regional manufacturing shifts and altered procurement and pricing strategies for vaccine stakeholders
The introduction of significant tariff changes in the United States in 2025 has had ripple effects across global vaccine supply chains, procurement strategies, and cost structures. Manufacturers that previously exported components or finished doses to and from the U.S. market encountered higher landed costs and uncertainty in production economics, prompting reassessments of global manufacturing footprints. In response, several producers accelerated regionalization efforts to mitigate exposure to tariff volatility, relocating critical stages of production closer to key demand centers to preserve price competitiveness and reduce transit complexity.Moreover, procurement officers and multinational purchasers adjusted contracting strategies to accommodate the new tariff landscape, placing greater emphasis on supplier diversification and clauses that share tariff-related risks. This shift influenced negotiating dynamics, with buyers seeking price-protection mechanisms and volume commitments that could stabilize supply. Concurrently, manufacturers reassessed their pricing and distribution models, exploring tiered pricing and targeted subsidies to maintain access in vulnerable populations while protecting margins in commercially viable markets.
The tariff environment also amplified the importance of supply chain transparency and traceability. Stakeholders invested in stronger supplier oversight and scenario planning to anticipate border-related disruptions. These investments aimed to preserve continuity of supply under dynamic trade policies. In sum, the tariff adjustments catalyzed a strategic reorientation toward regional manufacturing resilience, diversified sourcing, and contractual safeguards, all of which will influence how dengue vaccines are produced, priced, and delivered in the medium term.
Insightful segmentation analysis linking vaccine platforms channels dosage forms and demographic cohorts to operational choices and commercialization priorities
Segmentation-driven insights reveal critical levers for aligning product development and commercial strategies with end-user needs. Based on vaccine type, the landscape differentiates between live attenuated options and recombinant subunit candidates, and this split carries implications for immunogenic durability, cold chain sensitivity, and manufacturing complexity. Consequently, product teams should prioritize platform-specific scale-up plans and clinical communication strategies that reflect these intrinsic differences. Translating those biological attributes into programmatic decisions requires coordinated evidence-generation plans that compare safety and performance across demographic groups.Examining distribution channel segmentation uncovers distinct logistical and commercial considerations for hospital pharmacies, online pharmacies, and retail pharmacies. Hospital pharmacies often demand bulk supply agreements and integrated cold chain support, whereas online pharmacies require discrete parceling and robust delivery verification systems. Retail pharmacies, frequently the community-facing distribution points, emphasize patient education materials and point-of-care cold storage. These channel-specific needs necessitate tailored packaging, dosing formats, and commercial models to ensure consistent availability and optimal patient experience.
End user segmentation across clinics, hospitals, and research institutes highlights divergent procurement timelines and evidence requirements. Clinics often prioritize ease of administration and minimal storage burden, hospitals focus on formulary inclusion and institutional safety surveillance, and research institutes require access to investigational formulations and flexible sourcing. Aligning commercial strategies with these user types demands differentiated engagement plans and technically oriented support functions.
Dosage form segmentation into multi-dose vial, prefilled syringe, and single-dose vial formats shapes both operational workflows and acceptance among providers and patients. Multi-dose vials offer economies of scale but increase open-vial wastage risks and reconstitution training needs; prefilled syringes enhance safety and convenience yet raise per-dose production and cold chain considerations; single-dose vials balance wastage reduction with packaging and transport constraints. Decisions about which dosage forms to prioritize should weigh clinic throughput, cold chain capacity, and waste-management practices.
Finally, age group segmentation across adolescents, adults, and children requires age-tailored safety messaging, dosing strategies, and community engagement. Pediatric considerations often drive conservative regulatory approaches and influence immunization schedules, while adolescent and adult strategies can focus on workplace and school-based delivery opportunities. Integrating these segmentation lenses enables stakeholders to design targeted clinical programs, operational plans, and communication strategies that reflect both biological characteristics and healthcare delivery realities.
A comparative regional assessment showing how epidemiology regulatory environments and delivery capacity shape tailored strategies for dengue vaccine deployment
Regional dynamics exert powerful influence over vaccine deployment strategies, requiring nuanced approaches that reflect epidemiology, regulatory environments, and delivery infrastructure. In the Americas, national immunization programs combine centralized procurement with significant private-sector delivery channels, creating opportunities for hybrid public-private partnerships and school-based immunization campaigns. Policymakers and manufacturers in the region must navigate heterogeneous regulatory timelines and reimbursement models, and they benefit from early stakeholder engagement to synchronize clinical evidence with program rollout plans.In Europe, Middle East & Africa, regulatory sophistication is diverse and policy alignment varies widely. Some countries in this region maintain advanced regulatory pathways and robust pharmacovigilance systems, while others face resource constraints that impede rapid scale-up. Consequently, manufacturers often adopt regionally tailored regulatory strategies that include phased submissions, capacity-building support for national authorities, and investments in local cold chain enhancements. Engagement with multinational health agencies and regional procurement mechanisms can help smooth access across disparate jurisdictions.
Asia-Pacific exhibits a combination of high-burden areas and countries with strong manufacturing capabilities, creating both demand and supply-side opportunities. Several nations in this region have experience with vaccine manufacturing and export, which supports regional supply resilience. At the same time, densely populated urban centers and remote rural populations present distinct delivery challenges, necessitating creative distribution strategies that leverage community health workers, digital appointment systems, and decentralized cold chain logistics. Coordinating across the region requires sensitivity to national priorities, pricing constraints, and local health system capacities.
Across all regions, alignment between clinical evidence, regulatory acceptance, and practical delivery models is paramount. Regional strategies that integrate local stakeholder input, reinforce surveillance systems, and invest in targeted cold chain improvements are more likely to achieve high uptake and sustained protection against dengue.
A competitive dynamics overview illustrating how manufacturing partnerships licensing strategies and evidence-generation investments determine market leadership potential
Competitive dynamics in the dengue vaccine sector center on organizations that have advanced late-stage clinical programs, those establishing regional manufacturing capacity, and partners building robust pharmacovigilance and distribution capabilities. Some developers have prioritized platform differentiation to address specific programmatic needs, while others emphasize scalable manufacturing processes and flexible dosage formats. Strategic alliances between developers, contract manufacturers, and logistics providers have become commonplace, reflecting a recognition that clinical success must be complemented by operational excellence.Intellectual property strategies and licensing agreements play a decisive role in determining who controls manufacturing know-how and regional supply. Where technology transfer occurs, recipient manufacturers can accelerate local production, reducing time-to-supply and insulating local markets from trade disruptions. Conversely, limited licensing can concentrate supply and necessitate contractually complex procurement arrangements. Therefore, companies that proactively design equitable licensing and co-production models can strengthen their commercial and reputational positions.
In addition, leaders in this space are investing in real-world evidence collection and post-marketing safety systems to support regulatory relationships and payer confidence. Effective surveillance bolsters trust among clinicians and policymakers and facilitates adaptive policy decisions. Finally, organizations that offer comprehensive service bundles-combining clinical evidence, tailored packaging, and distribution support-stand to capture greater preferential inclusion in national immunization strategies. Strategic focus on manufacturing resilience, partnership ecosystems, and evidence-generation will differentiate successful competitors.
Practical and prioritized strategic actions for manufacturers and policymakers to strengthen manufacturing resilience and accelerate equitable vaccine rollout
Industry leaders should prioritize a set of actionable moves that bridge clinical promise and delivery practicality to accelerate public health impact. First, invest in regional manufacturing resilience to reduce exposure to trade volatility and to shorten lead times. Establishing local or regional production hubs, combined with validated technology transfer agreements, can mitigate tariff-related risks and enable faster response to outbreak spikes. In parallel, develop flexible packaging and multiple dosage-form strategies so that supply can be matched to the specific needs of clinics, hospitals, and community delivery programs.Second, deepen engagement with national health authorities and multilateral procurement bodies early in the product lifecycle. Collaborative policy development, including shared post-authorization evidence plans and phased procurement agreements, can smooth uptake and align expectations on safety monitoring. Third, design distribution strategies that reflect channel-specific requirements; support hospital pharmacies with integrated cold chain solutions, enable retail pharmacy participation through patient education tools, and adapt e-commerce logistics to ensure secure, temperature-controlled deliveries.
Fourth, embed robust pharmacovigilance and real-world evidence systems within launch plans to maintain regulatory trust and to inform iterative label expansions. Fifth, tailor communications and community engagement for age-group-specific concerns, emphasizing safety in pediatric contexts and convenience for adolescent and adult vaccination programs. Finally, fortify contractual terms with procurement partners to address tariff exposure and supply continuity, using scenario planning and shared-risk mechanisms to protect both suppliers and purchasers. These recommendations provide a pragmatic roadmap to translate vaccine innovation into sustainable, equitable protection.
A transparent multi-method research approach combining stakeholder interviews regulatory analysis and supply chain mapping to generate actionable strategic insights
This research synthesis draws on a multi-method approach combining literature review, stakeholder interviews, supply chain mapping, and regulatory document analysis to ensure a robust and actionable evidence base. Peer-reviewed publications, regulatory advisories, and public health guidance documents were examined to capture clinical, safety, and policy trends. In addition, structured interviews with senior leaders across manufacturing, procurement, and distribution provided qualitative insights into operational constraints, partnership models, and strategic priorities.Supply chain mapping exercises were used to identify critical nodes susceptible to trade disruptions and to evaluate mitigation strategies such as regionalization and dual sourcing. Regulatory document analysis focused on approval pathways, post-market obligations, and variations in labeling requirements across jurisdictions. Cross-validation of findings occurred through triangulation between primary interviews, public documentation, and technical white papers to ensure consistency and to surface areas of uncertainty that warrant further investigation.
Limitations include the dynamic nature of regulatory decisions and trade policies, which may evolve after the synthesis was completed; therefore, findings emphasize strategic implications rather than definitive predictions. Wherever possible, the methodology prioritized transparency and reproducibility, and stakeholders are encouraged to use the report's scenario frameworks to update assumptions as new data emerge. The combined methodological approach supports both high-level strategic conclusions and operationally relevant recommendations.
A concise conclusion emphasizing the necessity of integrated planning across science regulatory strategy manufacturing and distribution to deliver broad dengue protection
Dengue vaccine development has moved into a phase where scientific viability must be matched by delivery pragmatism to realize population-level benefits. The interplay between platform choice, dosage form, distribution channel, and demographic targeting determines whether clinical successes translate into accessible and sustainable immunization programs. Stakeholders who align evidence-generation with manufacturing resilience and tailored distribution will be better positioned to achieve meaningful public health outcomes.Trade policy shifts and regional differences in regulatory capacity underscore the need for flexible operational strategies and collaborative procurement mechanisms. Building regional production capabilities, investing in robust post-marketing surveillance, and designing channel-specific distribution plans will be central to maintaining supply continuity and optimizing uptake. Moreover, transparent licensing practices and technology transfer can expand manufacturing options and reduce dependence on single-source suppliers.
In conclusion, the path from candidate vaccines to broad protection requires integrated planning that balances scientific rigor with logistical feasibility. Decision-makers should adopt adaptive strategies that anticipate policy changes, prioritize equitable access, and commit to evidence-informed engagement with health authorities and communities. By doing so, they can turn recent technological progress into durable public health gains.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Dengue Vaccine Market
Companies Mentioned
- Biological E. Limited
- GeneOne Life Science
- GlaxoSmithKline (GSK) plc
- Johnson & Johnson Services, Inc.
- Medigen Vaccine Biologics
- Merck & Co. Inc.
- Novartis AG
- Sanofi Pasteur
- Serum Institute of India
- Sun Pharmaceutical Industries Ltd.
- Takeda Pharmaceutical Company
- Vabiotech
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 188 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
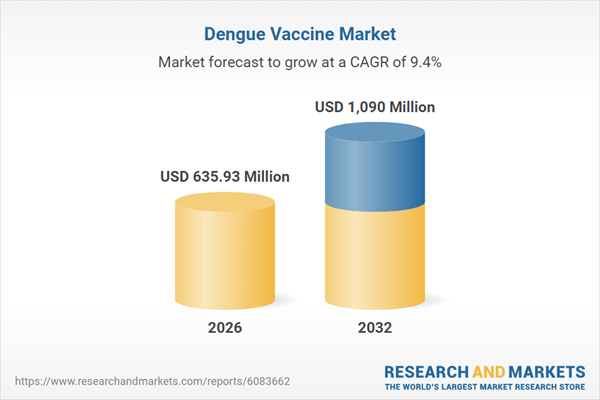
| Estimated Market Value ( USD | $ 635.93 Million |
| Forecasted Market Value ( USD | $ 1090 Million |
| Compound Annual Growth Rate | 9.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |