Speak directly to the analyst to clarify any post sales queries you may have.
How material innovation, clinical workflow demands, and procurement practices converge to redefine product selection and performance expectations in dental burs
The dental burs landscape sits at the intersection of material science, clinical practice evolution, and supply chain modernization. Clinicians rely on burs to deliver precision in cavity preparation, implantology, oral surgery, and orthodontics, while manufacturers pursue improvements in durability, cutting performance, and biocompatibility. Innovations in ceramics, carbide formulations, and diamond coatings have prompted clinicians and procurement teams to re-evaluate product selection criteria, emphasizing cycle time, patient comfort, and sterilization compatibility.
Concurrently, channels of distribution and purchasing behaviors have shifted. Dental clinics and hospitals balance cost containment with the imperative to adopt devices that reduce procedure time and improve clinical outcomes. Procurement teams increasingly demand evidence of consistency and traceability, and regulatory expectations on device labeling and sterility validation have tightened. These forces combine to create a dynamic environment where product differentiation rests on demonstrable performance advantages, operational value, and alignment with evolving clinical protocols.
Understanding this environment requires a layered view that spans raw material properties, shank geometries, grit selections, and end-use requirements. Integrating technical specifications with end-user workflows reveals how small design choices translate into meaningful gains in efficiency and patient experience, and it underscores the importance of targeted, clinically informed product development.
Material engineering, regulatory rigor, digital dentistry integration, and supply chain resilience are reshaping competitive advantage across the dental bur ecosystem
The dental bur sector is undergoing transformative shifts driven by advances in materials engineering, regulatory emphasis on device validation, and the maturation of digital dentistry workflows. Improvements in carbide metallurgy, the wider adoption of engineered ceramics, and refinement of diamond-coating techniques have raised the baseline for durability and cutting fidelity. These material improvements support faster cutting speeds and reduced need for frequent instrument changes, which in turn influence clinical scheduling and throughput.
At the same time, regulatory frameworks now place greater emphasis on traceability and sterilization validation, pressing manufacturers to standardize labeling and invest in documentation systems. This regulatory tightening not only protects patients but also elevates the expectations of institutional buyers who seek partners capable of supporting audit and compliance requirements. Meanwhile, the growth of CAD/CAM and digital planning tools creates new touchpoints between restorative planning and the selection of bur geometries optimized for computer-assisted workflows.
Supply chain resilience and distribution agility have also become strategic differentiators. Manufacturers that invest in localized manufacturing or regional warehousing can better navigate procurement constraints and reduce lead times, while those that offer transparent supply chain information reinforce buyer confidence. Collectively, these shifts reposition competitive advantage around integrated capabilities: material performance, regulatory readiness, digital compatibility, and supply chain reliability.
Trade policy adjustments in 2025 compel supply chain realignment, sourcing diversification, and pricing strategies that influence procurement and supplier partnerships
United States tariff adjustments announced for 2025 introduce a new variable into procurement planning and strategic sourcing for dental instrumentation. Tariff changes alter landed cost dynamics and can incentivize reconfiguration of supplier networks, particularly for product lines sensitive to raw material and import costs. For manufacturers and distributors operating global supply chains, tariffs increase the importance of strategic localization, tariff engineering, and contractual flexibility to protect margins and stabilize pricing for end users.
These policy shifts also affect inventory strategies. Healthcare providers and procurement teams may reevaluate stocking practices to mitigate short-term price volatility, while suppliers will look to secure longer-term contracts or diversify manufacturing footprints to maintain competitive pricing. In parallel, tariff-driven cost pressures can accelerate innovation in product design, as manufacturers pursue performance enhancements that justify price adjustments and reinforce perceived value. Clinical decision-makers may respond by scrutinizing total cost of ownership, factoring in bur longevity, procedural efficiency, and sterilization lifecycles when evaluating options.
Finally, tariff changes can catalyze strategic partnerships between regional manufacturers and distributors to ensure continuity of supply. Organizations that proactively communicate supply chain adjustments and provide comparative performance data will better retain institutional customers. The net effect is a marketplace where trade policy acts as a catalyst for structural change in sourcing, pricing strategy, and supplier relationships.
Granular segmentation across material classes, shank geometries, grit profiles, clinical applications, and end-use settings reveals distinct product and commercialization imperatives
Segment-level characteristics reveal differentiated value propositions that inform product development priorities and go-to-market strategies. Based on Material, market is studied across Carbide Burs, Ceramic Burs, Diamond Burs, and Steel Burs; each material class brings unique attributes: carbide variants emphasize toughness and cutting edge retention, ceramics can offer exceptional wear resistance with biocompatible finishes, diamond burs provide unmatched abrasiveness for hard tissue work, and steel burs serve cost-sensitive procedural needs. These material distinctions translate into clinical choices driven by tissue type, desired finish, and rotational speed.
Based on Shank Type, market is studied across Friction Grip and Right Angle; shank geometry influences handpiece compatibility, torque transfer, and operator ergonomics, with friction grip shanks favored in high-speed handpieces and right-angle configurations used in contra-angle applications. Based on Grit Size, market is studied across Coarse, Fine, and Medium; grit selection determines surface finish, aggressiveness of cutting, and heat generation, and clinicians balance these factors to achieve clinical objectives while minimizing patient discomfort. Based on Application, market is studied across Cavity Preparation, Implantology, Oral Surgery, and Orthodontics; each clinical domain demands specific design features, such as cutting profiles for bone versus enamel, which shapes R&D focus and sterilization protocols. Based on End-use, market is studied across Dental Clinics and Hospitals; purchasing behavior, volume requirements, and clinical protocol differences between ambulatory clinics and hospital operating rooms drive distinct product configurations and service offerings.
Understanding segmentation at this level enables stakeholders to link technical specifications to clinical outcomes. Product roadmaps should prioritize cross-compatibility, ergonomics, and evidence-based performance claims, while commercial strategies must differentiate offerings by the clinical value they deliver in specific procedural contexts.
Regional market drivers and regulatory diversity require tailored regulatory strategies, localized distribution, and clinician-focused adoption programs across global regions
Regional dynamics shape demand patterns, regulatory expectations, and distribution models in ways that warrant differentiated regional strategies. In the Americas, buyers prioritize rapid delivery, documented sterilization processes, and partnerships that ensure service continuity across urban and rural clinics; the region's established dental networks emphasize clinical evidence and cost-effectiveness, prompting suppliers to offer validated performance data and responsive logistics. Europe, Middle East & Africa presents a mosaic of regulatory frameworks and procurement models, where compliance with varied national standards and the ability to support institutional tenders differentiate suppliers, and where sustainability and recyclability concerns increasingly influence purchasing committees.
Asia-Pacific displays fast-evolving clinical infrastructure and rising adoption of advanced dentistry techniques, driving demand for high-performance materials and compatible digital workflows. Regional manufacturing capacity expansion in Asia-Pacific supports localized supply, but buyers in that region also seek internationally certified products and evidence of consistent quality. Across all regions, connectivity between manufacturers and professional associations, continuing education programs, and clinical training initiatives helps accelerate adoption of new bur technologies by demonstrating clinical advantages and safe handling practices.
Consequently, companies should tailor product portfolios, regulatory strategies, and distribution partnerships to regional preferences, investing in localized training and documentation to support adoption and long-term relationships with institutional buyers.
Competitive differentiation is driven by material and coating innovation, clinical validation partnerships, and operational excellence that support institutional procurement
Competitive dynamics in the dental bur landscape reflect a mix of specialized manufacturers, precision toolmakers, and medical device firms that combine engineering expertise with clinical validation. Leaders differentiate through consistent quality control, invested R&D in materials and coatings, and the ability to support institutional compliance requirements. Companies that provide comprehensive technical documentation, sterilization guidance, and training resources strengthen customer trust and reduce barriers to procurement by large dental networks and hospital systems.
Partnerships with dental schools, continuing education providers, and clinical opinion leaders play an outsized role in shaping clinician preferences. Firms that enable hands-on evaluation through workshops or simulation labs accelerate clinician confidence and can shorten adoption cycles. Additionally, firms that integrate digital tools such as product traceability apps or online ordering platforms create stickiness with procurement teams by simplifying inventory management and reordering processes.
Operational excellence remains critical: production consistency, clear quality metrics, and reliable distribution underpin long-term relationships. Strategic investments in manufacturing standardization, ISO certifications, and transparent supply chain practices position companies to compete effectively across clinic and hospital segments, while targeted product differentiation addresses specific clinical use-cases and end-user workflows.
Integrate product innovation, regional manufacturing resiliency, clinician training, and digital procurement tools to build durable competitive advantage and institutional partnerships
Industry leaders should pursue an integrated agenda that aligns product innovation with supply chain resilience and clinician engagement to create defensible competitive advantage. Prioritize R&D that advances material properties and coating processes to extend tool life and reduce procedural time, while simultaneously investing in sterilization compatibility and lab-validated performance data that resonate with institutional buyers. These technical improvements should be complemented by transparent quality systems and traceability tools that simplify audits and support buyer compliance requirements.
Expand regional manufacturing or warehousing to mitigate tariff exposure and shorten lead times, and develop multi-sourcing strategies for critical inputs to reduce vulnerability to trade policy shifts. Engage clinicians through comprehensive training, simulation workshops, and evidence dissemination so that performance claims convert into practice change. Commercially, offer flexible procurement models such as contractual pricing, bundled service offerings, or managed inventory solutions to accommodate the differing purchasing practices of dental clinics versus hospitals.
Finally, invest in digital tools that facilitate ordering, product verification, and lifecycle tracking, thereby enhancing customer experience and operational efficiency. By combining technical superiority, regulatory readiness, and customer-centric commercial models, companies will be better positioned to capture long-term institutional partnerships and support clinicians in delivering improved patient outcomes.
A mixed-method research approach combining clinician interviews, procurement input, regulatory review, and technical validation to ensure actionable and credible insights
The research methodology underpinning these insights integrates primary interviews with clinicians, procurement specialists, and industry technologists, alongside secondary analysis of regulatory guidelines, manufacturing best practices, and clinical literature. Primary engagements emphasized first-hand experiences with bur performance across common procedures, sterilization workflows, and procurement decision drivers, enabling qualitative validation of material and design hypotheses. Secondary sources provided context on regulatory expectations, material science advances, and supply chain practices relevant to the sector.
This mixed-method approach emphasizes triangulation: clinician observations were cross-checked with product specification reviews and manufacturing process audits, and procurement feedback was aligned with observed distribution and warehousing practices. The methodology prioritized representative sampling across end-user types to capture distinctions between dental clinics and hospital procurement models, and it examined product performance across the defined segmentation categories to ensure relevance to both design and commercial stakeholders.
Where appropriate, technical performance claims cited in manufacturer documentation were validated through independent laboratory literature and expert corroboration. The combination of practitioner insight, technical appraisal, and supply chain analysis yields pragmatic recommendations that reflect operational realities and clinical priorities.
Translating material and design innovation into validated clinical advantage requires coordinated R&D, regulatory readiness, and buyer-centric distribution strategies
The dental bur sector is poised to evolve through incremental material advances, tighter regulatory expectations, and shifting procurement dynamics driven by trade policy and regional development. Success will favor organizations that link technical innovation with demonstrable clinical benefits and resilient supply chain strategies. Clinicians will continue to prioritize tools that reduce procedure time and improve patient outcomes, while institutional buyers will demand traceability, sterilization assurance, and transparent supply arrangements.
Looking ahead, firms that invest in clinician education, localized operational capacity, and digital tools for procurement will better align with buyer needs and enhance adoption rates. Cross-functional collaboration between R&D, regulatory, and commercial teams will prove essential to translate material and design innovations into validated clinical benefits. By focusing on performance, compliance, and customer experience, stakeholders can navigate policy changes and regional variations to deliver practical solutions that support modern dental practice.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Dental Burs Market
Companies Mentioned
The key companies profiled in this Dental Burs market report include:- 3M Company
- American Orthodontics Corporation
- AXIS Dental Sàrl
- Beyes Dental Canada Inc.
- Bicon, LLC
- Brasseler USA
- Buffalo Dental Manufacturing Co. Inc.
- Coltene Holding AG
- Danaher Corporation
- DentalEZ, Inc.
- Dentsply Sirona Inc.
- Diatech
- Dynaflex Private Limited
- Kerr Corporation
- Komet USA
- Mani, Inc.
- Microcopy
- Midmark Corporation
- MIS Implants Technologies Ltd.
- Nakanishi Inc.
- Patterson Dental
- Prima Dental Manufacturing Ltd.
- SHOFU Inc.
- Straumann AG
- SUPER TOOL co. ltd.
- SYNDENT Tools Co., Ltd.
- Tri Hawk Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 187 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
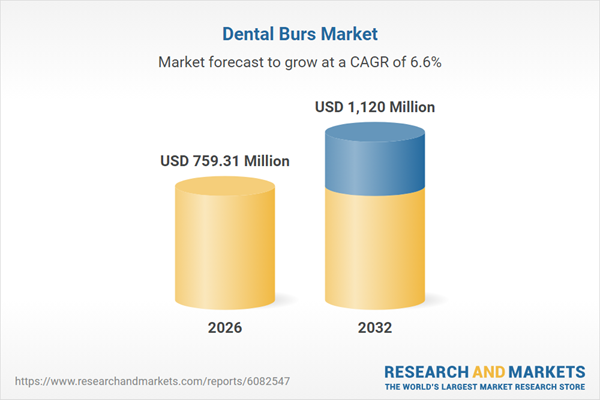
| Estimated Market Value ( USD | $ 759.31 Million |
| Forecasted Market Value ( USD | $ 1120 Million |
| Compound Annual Growth Rate | 6.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 28 |