Speak directly to the analyst to clarify any post sales queries you may have.
The artificial disc replacement market is redefining spinal care by aligning advanced device designs and clinical priorities with the operational needs of hospitals, ambulatory centers, and spine specialists. Senior decision-makers must understand shifting adoption patterns, technology progress, and external pressures shaping this market’s future.
Market Snapshot
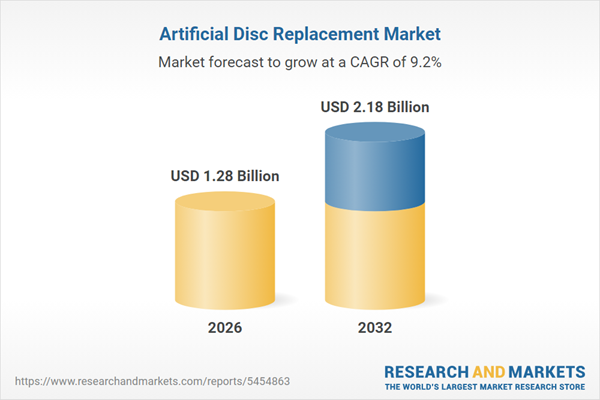
The Artificial Disc Replacement Market experienced robust expansion, rising from USD 1.18 billion in 2025 to USD 1.28 billion in 2026. It is projected to sustain a compound annual growth rate (CAGR) of 9.15%, reaching USD 2.18 billion by 2032.
This sustained momentum is rooted in the transition of artificial disc replacement from a specialized procedure to a core solution within contemporary spine care. The surge in demand reflects collaborative innovation among device developers, payers, and care providers, together driving clinical integration and commercial attractiveness.Scope & Segmentation
- Applications: Addresses both Cervical (with Multi Level and Single Level sub-divisions) and Lumbar (likewise Multi Level and Single Level), recognizing different surgical goals and biomechanical requirements.
- Constraint Types: Encompasses Constrained, Semi-Constrained, and Unconstrained Designs, each supporting unique clinical philosophies on motion control and segmental stability.
- Material Types: Examines Metal on Metal and Metal on Polymer constructs, highlighting the implications for imaging compatibility, wear resistance, and revision strategy.
- End User Channels: Segments the market by Ambulatory Surgical Centers, Hospitals, and Specialized Spine Centers for precise targeting of procedural complexity and innovation adoption environments.
- Geographic Regions: Includes Americas, EMEA, and Asia-Pacific, referencing diverse regulatory frameworks, clinical infrastructures, and procurement models shaping local adoption practices.
Key Takeaways
- Surgical innovation is accelerating, with evolving device designs supporting both traditional and minimally invasive workflows. Surgeons now balance motion preservation, ease of revision, and integration with navigation and robotics.
- Device manufacturers advance mechanical performance through improved biomaterials, bearing surfaces, and instrument systems designed for reproducibility and simplified adoption.
- Procurement strategies are adapting to prioritize value-driven selection, leveraging robust comparative evidence and contracting for cost stability and supply chain resilience.
- Payer frameworks increasingly require real-world data and demonstrable clinical outcomes before approving broader reimbursement, making evidence generation a strategic priority.
- Adoption patterns are highly segmented; ambulatory centers expand single-level cases, hospitals specialize in complex procedures, while specialized spine centers drive innovation by adopting new clinical paradigms.
- Consistent post-market support, surgeon training, and digital registry tools are key for vendors aiming to differentiate and foster long-term relationships with health systems.
Tariff Impact on Supply Chain & Procurement
The introduction of new tariff regulations in 2025 for artificial disc replacement components and finished products has led manufacturers to reconsider sourcing geographies and insourcing options. Hospitals and spine centers now intensify supplier vetting and secure longer-term contracts to ensure price stability and inventory continuity. Facility administrators, in turn, review inventory and reimbursement agreements to mitigate financial volatility without compromising device access. These dynamics accelerate vertical integration, contract partnerships, and regional manufacturing as organizations aim to sustain clinical flexibility and supply assurance.
Methodology & Data Sources
This report employs a mixed-methods research approach, integrating structured interviews with orthopedic and neurosurgical leaders, hospital procurement experts, and device engineers. Site observations across ambulatory surgical centers, specialty spine clinics, and hospitals complement the interviews by providing real-world workflow insights. Secondary sources include peer-reviewed clinical literature, regulatory filings, device registries, and patent analyses, ensuring comprehensive and validated findings.
Why This Report Matters
- Provides actionable insight into the intersection of clinical need, technology advancement, and procurement strategy in the artificial disc replacement market.
- Equips decision-makers to anticipate supply chain disruptions, regulatory shifts, and evolving reimbursement criteria with clarity and operational context.
- Guides investments in evidence generation, product development, and commercial models aligned to region, user channel, and care complexity.
Conclusion
Artificial disc replacement is entering a pivotal phase of clinical, operational, and commercial transformation. Stakeholders who prioritize clinically validated solutions, supply chain resilience, and responsive commercial strategies are well positioned to unlock enduring market opportunities in spinal care.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Artificial Disc Replacement Market
Companies Mentioned
The key companies profiled in this Artificial Disc Replacement market report include:- AxioMed LLC
- B. Braun Melsungen AG
- Centinel Spine, LLC
- Globus Medical, Inc.
- HIGHRIDGE MEDICAL
- Johnson & Johnson
- Medtronic plc
- NuVasive, Inc.
- Orthofix Medical Inc.
- Spinal Elements, Inc.
- Stryker Corporation
- SYNERGY SPINE SOLUTIONS INC.
- Zimmer Biomet Holdings, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 190 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 1.28 Billion |
| Forecasted Market Value ( USD | $ 2.18 Billion |
| Compound Annual Growth Rate | 9.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 14 |