Speak directly to the analyst to clarify any post sales queries you may have.
A strategic introduction outlining clinical imperatives, technological evolution, and operational priorities that define modern elbow fixation systems
The elbow fixation system market occupies a critical intersection of orthopedic trauma care, implant technology, and evolving care delivery models. Clinicians confront a wide spectrum of fracture patterns and soft tissue considerations that demand implants and fixation strategies capable of restoring anatomy, preserving joint function, and enabling early mobilization. Concurrently, device developers and clinical service providers must navigate tighter operating room workflows, growing demand for same‑day procedures, and an intensified focus on value that links clinical outcomes to total episode costs.Innovation in implant geometry, fixation mechanics, and biomaterials is redefining surgical options, while advances in imaging, intraoperative navigation, and precontouring techniques are improving fit and fixation. Regulatory scrutiny and reimbursement dynamics continue to shape adoption pathways, prompting manufacturers to emphasize clinical evidence generation and surgeon education as key differentiators. In parallel, supply chain resilience and manufacturing agility have moved to the forefront as stakeholders prioritize continuity, quality, and cost containment.
Taken together, these factors create a complex strategic environment where clinical efficacy, device versatility, and commercial execution determine success. This introduction frames the report’s analytical approach, emphasizing clinical context, technology trajectories, and operational levers that will guide decision‑makers as they assess product portfolios, commercialization strategies, and partnerships across the elbow fixation ecosystem.
An analysis of converging clinical, technological, and care delivery shifts that are redefining product design, adoption pathways, and commercial models across elbow fixation
The elbow fixation landscape is undergoing multiple transformative shifts that are reshaping clinical practice and commercial strategy. Minimally invasive and tissue‑sparing approaches are gaining momentum, encouraging designs that reduce dissection while providing stable fixation; simultaneously, locking plate geometry and precontoured solutions are responding to the need for anatomical conformity and reproducible fixation across complex fracture patterns. Material science improvements, including refined titanium alloys and advanced polymers, are enabling strength and biocompatibility tradeoffs that expand surgeon choice.In addition, digital technologies are increasingly embedded within the surgical pathway. Preoperative planning and patient‑specific models, intraoperative imaging and navigation, and instrument trays optimized for efficiency are collectively reducing procedure time and variability. These technological shifts are complemented by changes in care delivery: the growth of ambulatory surgical centers and the transfer of lower‑acuity procedures out of traditional hospitals are influencing device packaging, sterilization workflows, and distribution strategies. As a result, manufacturers and health systems are prioritizing modular product portfolios, streamlined supply chains, and evidence generation to validate shorter lengths of stay and improved functional recovery.
Consequently, the competitive terrain is being defined not only by implant performance but also by the ability to integrate clinical education, data capture, and logistical support into comprehensive solutions that address the full episode of care. Stakeholders who align product design, digital enablement, and channel strategy will be best positioned to translate these shifts into sustainable clinical and commercial gains.
A practical assessment of how United States tariff adjustments in 2025 reshaped supply chain resilience, sourcing strategies, and commercial pricing dynamics across the value chain
Policy changes affecting tariffs in the United States in 2025 have altered multiple facets of the elbow fixation value chain, compelling device manufacturers, distributors, and providers to reexamine sourcing, pricing, and inventory strategies. Increased import duties on certain medical components have raised the cost basis for implants and instrumentation built with imported raw materials or subassemblies, prompting procurement teams to reassess supplier contracts and to explore nearshoring and domestic manufacturing solutions to mitigate exposure. At the same time, longer lead times and elevated shipping costs have reinforced the imperative for robust inventory management and dynamic safety stock policies.Manufacturers have responded through several pragmatic adjustments. First, some suppliers prioritized dual sourcing and qualification of alternate vendors to preserve continuity while maintaining regulatory compliance. Second, value engineered designs that maintain clinical performance while reducing dependency on tariff‑sensitive inputs have been accelerated. Third, commercial teams adopted more transparent pricing communications with customers to preserve trust while explaining cost drivers and potential contractual implications. These responses were accompanied by renewed attention to regulatory documentation, since any material or supplier changes necessitate clear traceability and, in some cases, supplemental evidence to reassure clinicians and purchasers.
Overall, the cumulative effects of tariff changes extend beyond immediate cost pressures: they have catalyzed strategic shifts toward supply chain resilience, product modularity, and localized production capability. Organizations that proactively diversified sourcing, optimized manufacturing footprints, and aligned commercial messaging with clinical value were able to navigate the policy environment with fewer disruptions and preserve market access in complex procurement environments.
Segment intelligence that links product modalities, clinical indications, care settings, material choices, and distribution routes to actionable strategic priorities and commercialization levers
Segment analysis reveals differentiated drivers and constraints across product types, clinical indications, end users, materials, and distribution models. Product diversity spans external fixation, intramedullary nails, plate and screw fixation, and suture anchors, with plate and screw fixation further differentiated into locking plates and precontoured plates; each modality addresses specific biomechanical needs and surgical philosophies, and device selection is often dictated by fracture morphology and surgeon training. Indication segments include distal humerus fracture, lateral epicondyle fracture, olecranon fracture, and radial head fracture, each of which presents unique anatomical and functional restoration challenges that influence implant design, fixation strategy, and postoperative rehabilitation protocols.End‑user dynamics vary substantially between ambulatory surgical centers, hospitals, and specialty clinics. Ambulatory environments prioritize streamlined instrumentation, single‑use or easily reprocessed kits, and products validated for efficient turnover, whereas hospitals maintain broader inventories to support complex trauma and revision procedures. Specialty clinics focus on niche indications and may partner closely with manufacturers for training and procedure standardization. Material selection among polymer, stainless steel, and titanium options shapes implant performance, corrosion resistance, and imaging artifact profiles; polymer components are attractive for certain low‑load applications, stainless steel offers cost advantages and robustness, and titanium balances strength with biocompatibility and reduced MRI artifact.
Distribution channel strategies encompass direct sales, distributor sales, and online sales, each with distinct implications for margin structure, clinical support, and geographic reach. Direct sales models enable tighter clinical engagement and tailored training but require substantial field resources. Distributor networks offer extended market penetration and logistical support, particularly in regions with fragmented hospital purchasing. Online sales channels are emerging for consumables and instrumentation accessories, accelerating procurement cycles and enabling smaller providers to access specialized products. Integrating these segmentation insights into product development, pricing, and go‑to‑market planning will be critical to aligning offerings with the behavioral, operational, and economic needs of each customer cohort.
A regionally differentiated overview that connects regulatory frameworks, clinical adoption pathways, and supply chain considerations across the Americas, EMEA, and Asia‑Pacific
Regional dynamics shape the adoption curves and operational priorities for elbow fixation systems across the Americas, Europe, Middle East & Africa, and Asia‑Pacific, each region presenting distinct regulatory ecosystems, reimbursement frameworks, and provider structures. In the Americas, high procedural volumes and an advanced network of ambulatory surgical centers support the adoption of minimally invasive implants and devices that reduce operative time, whereas procurement cycles and payer negotiations emphasize outcomes and total cost of care. In contrast, Europe, Middle East & Africa presents a mosaic of regulatory pathways and reimbursement mechanisms, with some markets prioritizing national tenders and others emphasizing commercial relationships and clinician preference, creating a complex market access environment.The Asia‑Pacific region combines rapid infrastructure expansion, growing surgical capacity, and a rising emphasis on localized manufacturing and technology transfer. Regional manufacturing hubs and supply chain nodes in Asia influence global sourcing decisions, while local regulatory authorities increasingly require region‑specific clinical data or post‑market surveillance. Across all regions, clinical education and demonstration of functional outcomes remain central to adoption, but the modalities for engagement differ: some markets prioritize hands‑on cadaver labs and peer‑to‑peer training, while others rely on digital platforms and virtual simulation. As a result, companies must tailor their market entry and commercialization strategies to align with regional clinical priorities, regulatory expectations, and distribution realities to maximize uptake and operational efficiency.
A competitive assessment that emphasizes product differentiation, evidence generation, and integrated perioperative support as decisive factors shaping company positioning
Competitive dynamics in the elbow fixation space are driven by differentiated product portfolios, depth of clinical evidence, and the capacity to deliver comprehensive perioperative support. Established implant manufacturers focus on product refinements such as advanced locking mechanisms, anatomically contoured plates, and integrated instrumentation that reduce intraoperative variability. At the same time, smaller innovators and specialist firms are advancing niche solutions-such as low‑profile suture anchors, hybrid fixation constructs, and disposable instrumentation-that target clear procedural pain points and enable faster turnover.Across the industry, strategic priorities include clinical trial investment, surgeon engagement programs, and partnerships with health systems to capture real‑world performance data. Manufacturing capability and supply chain transparency have become important competitive differentiators as purchasers demand traceability and dependable lead times. Additionally, companies that integrate digital tools for preoperative planning, outcome tracking, and remote training create stickier relationships with customers by reducing onboarding friction and improving measurable outcomes. Collaborations between device developers, digital health firms, and academic centers are accelerating evidence generation and establishing clinical pathways that favor integrated solutions.
Overall, competitive success hinges on the ability to marry technical excellence with scalable commercial models and to demonstrate consistent clinical utility across diverse care settings. Organizations that can align product innovation with robust clinical validation and responsive supply chains will maintain strategic advantage in an increasingly outcome‑driven procurement environment.
Practical, prioritized recommendations that address product modularity, supply chain resilience, channel strategy, and evidence generation to accelerate adoption and protect margins
Industry leaders should pursue a set of targeted, actionable priorities to capitalize on clinical demand while mitigating operational risk. First, invest in modular implant platforms and scalable instrumentation that accommodate a range of fracture patterns while reducing tray complexity and sterilization burden. Second, prioritize clinician training and evidence collection that link implant choice to meaningful functional outcomes; embed outcome measurement into commercial programs to build credibility with payers and hospital procurement teams. Third, diversify supply chains through dual sourcing, nearshoring, or contract manufacturing to reduce tariff and logistics exposure and to shorten lead times for high‑demand components.Fourth, adopt a channel‑hybrid approach that combines direct sales for high‑touch, complex procedures with distributor partnerships and online channels for consumables and standardized kits. Fifth, accelerate adoption of advanced materials and additive manufacturing where clinical benefit and supply advantages are demonstrable, while ensuring rigorous regulatory traceability. Sixth, engage proactively with regulatory bodies and hospital procurement to align on post‑market surveillance needs and value demonstration, thus smoothing market access. Finally, deploy digital enablers for preoperative planning, remote training, and outcomes capture to strengthen customer relationships and to create differentiated service offerings that extend beyond the implant itself.
Taken together, these actions will help organizations drive adoption, protect margins, and deliver measurable clinical value in an increasingly competitive and regulated environment.
A rigorous mixed‑methods research approach combining primary clinician input, regulatory and clinical evidence review, and supply chain mapping to support actionable strategy
The research underpinning this analysis rests on a mixed‑methods approach that integrates primary qualitative input, systematic literature review, and supply chain mapping to ensure robust, actionable findings. Primary research included structured interviews with orthopedic surgeons across trauma and sports disciplines, device procurement managers, operating room leaders, and clinical educators to capture procedural preferences, unmet needs, and adoption barriers. These insights were synthesized with a targeted review of regulatory submissions, clinical trial registries, device safety communications, and peer‑reviewed literature to validate performance claims and to identify emergent technological trends.Complementing the clinical and regulatory perspective, supply chain and commercial channel analysis examined manufacturing footprints, raw material sourcing, and distribution arrangements to assess vulnerability to policy shifts and logistical constraints. Segmentation matrices were developed to align product features with clinical indications and end‑user requirements, and these matrices were stress‑tested through scenario analysis to evaluate strategic options under alternative operational conditions. Finally, all findings were subjected to triangulation and peer review by subject‑matter experts to ensure methodological rigor and practical relevance for commercial and clinical stakeholders.
A concise conclusion synthesizing clinical priorities, innovation opportunities, and operational imperatives that will determine competitive success in elbow fixation
In conclusion, the elbow fixation ecosystem is poised at an inflection point where clinical need, material innovation, digital enablement, and distribution strategy converge to redefine how fractures are treated and how devices are brought to market. The imperative for implants that balance anatomical conformity, biomechanical stability, and operational efficiency is stronger than ever, and stakeholders must align product development with the realities of ambulatory care growth and tightening procurement scrutiny. Tariff adjustments and supply chain pressures have accelerated strategic planning around sourcing and manufacturing, underscoring the value of resilient, diversified operations.Key opportunities lie in modular plate systems, refined suture anchoring solutions, and integration of digital tools that enhance preoperative planning and outcomes tracking. Equally important is the need for evidence generation and clinician education to validate new approaches and to support broader adoption across hospitals, ambulatory surgical centers, and specialty clinics. By pursuing targeted investments in materials, expanding distribution flexibility, and embedding outcome measurement into commercial programs, organizations can translate technical advances into measurable improvements in patient care and operational performance.
Ultimately, success will be determined by the ability to combine clinical credibility with supply chain agility and customer‑centric commercialization, thereby delivering implants and services that meet the evolving demands of providers and payers alike.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Elbow Fixation System Market
Companies Mentioned
The key companies profiled in this Elbow Fixation System market report include:- Amitex Agro Product Pvt. Ltd.
- Archer Daniels Midland Company
- Baker Commodities, Inc.
- BASF SE
- Botanic Innovations, LLC
- BRF S.A.
- Bunge Global SA
- CREMER OLEO GmbH & Co. KG
- Darling Ingredients Inc.
- Godrej Agrovet Limited
- Influx Holding Sdn. Bhd.
- Jacob Stern & Sons, Inc.
- Koninklijke DSM N.V.
- Luzar Trading S.A.
- Narmadaraj Industries Limited
- Nature Power Agrotech Pvt Ltd
- Olenex Sàrl
- Om Shanti Enterprises
- Omega Protein Corporation
- Ridley Corporation Limited
- Royal Flour Mills (Pvt) Ltd.
- Saria Limited
- Susheela Group
- Viterra Limited
- Wilmar International Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
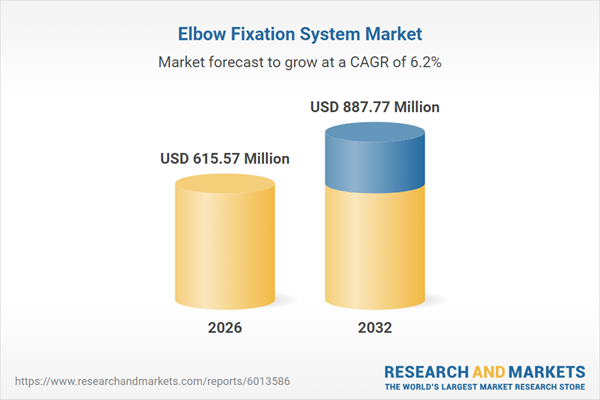
| Estimated Market Value ( USD | $ 615.57 Million |
| Forecasted Market Value ( USD | $ 887.77 Million |
| Compound Annual Growth Rate | 6.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |