Speak directly to the analyst to clarify any post sales queries you may have.
A concise yet comprehensive orientation to endoprosthesis dynamics, highlighting clinical priorities, materials innovation, and procurement implications
Introduction to the contemporary endoprosthesis landscape and the converging forces reshaping clinical and commercial decision-making
Endoprostheses occupy a central role at the intersection of surgical innovation, biomaterials science, and evolving care delivery models. Recent years have seen implantable devices transition from purely mechanical solutions to integrated clinical assets that influence perioperative workflows, postoperative rehabilitation, and long-term patient outcomes. As a result, stakeholders must evaluate devices not only on biomechanical performance but also on lifecycle economics, compatibility with minimally invasive techniques, and alignment with value-based care objectives.Overlaying technological progress are changing clinician preferences and patient expectations. Surgeons increasingly prioritize modularity, ease of revision, and implants compatible with advanced imaging and navigation systems. Concurrently, patients demand faster recovery, lower complication risk, and implants that support higher post-surgical activity levels. These dynamics are driving manufacturers to invest in novel materials, surface treatments, and design optimizations that reduce wear, improve osseointegration, and simplify revision procedures.
In parallel, regulatory and procurement frameworks have grown more sophisticated, placing greater emphasis on clinical evidence, real-world performance, and supply chain resilience. This combination of clinical, technological, and policy drivers creates a complex environment for decision-makers. Consequently, an integrated perspective that bridges product innovation, clinical workflows, and commercial strategy is essential for institutions, manufacturers, and investors seeking to navigate the endoprosthesis domain effectively.
Key transformative trends reshaping product development, clinical adoption, and commercial models in the endoprosthesis ecosystem
Transformational shifts altering the competitive and clinical environment for endoprosthetic devices across care settings
The endoprosthesis sector is undergoing transformative shifts that recalibrate competitive boundaries and clinical practice. First, material science breakthroughs and additive manufacturing methods are enabling customization at scale, which in turn affects inventory models and surgical planning. Manufacturers increasingly offer patient-specific geometries and porous architectures that promote biological fixation, while hospitals and surgical centers reassess inventory and sterilization workflows to accommodate these modular approaches.Second, the proliferating use of digital tools, including surgical navigation, intraoperative imaging, and preoperative planning software, has elevated device selection criteria. Implants that integrate seamlessly with digital ecosystems gain preference because they reduce intraoperative variability and support reproducible outcomes. At the same time, data captured through these systems fuels post-market surveillance and comparative effectiveness analyses, which influence purchasing committees and reimbursement decisions.
Third, care delivery is shifting toward ambulatory and outpatient settings for a growing subset of procedures. This migration prompts device manufacturers to emphasize ease of implantation, reduced operative time, and simplified instrument sets to meet the constraints of ambulatory surgical centers-whether free-standing or hospital-owned. As a result, product development now balances advanced functionality with operational simplicity to suit diverse end users.
Finally, payer and policy trends emphasize value-based procurement and long-term outcome metrics over episodic cost reductions. Accordingly, stakeholders must demonstrate not only initial implant performance but also durability, revision burden, and contribution to overall care pathway efficiency. Collectively, these shifts demand that industry participants align R&D, clinical evidentiary strategies, and commercial models to remain competitive and clinically relevant.
Operational, procurement, and strategic consequences of U.S. tariff measures on endoprosthesis supply chains and long-term industry positioning
Cumulative implications of proposed United States tariffs on medical device imports and the operational repercussions for manufacturers and providers
Proposed tariffs in the United States introduce a new layer of complexity to global supply chains for implantable devices and associated components. Manufacturers with international production footprints must reassess sourcing strategies, negotiate supplier contracts, and evaluate the viability of nearshoring or onshoring critical manufacturing steps to mitigate tariff exposure. These decisions influence lead times, production costs, and the capacity to respond to sudden demand shifts while preserving quality standards.Healthcare providers and purchasing organizations must also adapt procurement practices in response to cost inflation risks and potential product substitution pressures. Hospitals may face trade-offs between retaining incumbent implants with established clinical track records and adopting locally manufactured alternatives that can offer price relief. In certain cases, clinical committees will need to weigh operational considerations such as inventory diversification and vendor consolidation against clinical performance and surgeon preference.
Importantly, tariffs catalyze strategic responses beyond price adjustments. Manufacturers may accelerate localization of intellectual property-sensitive processes or form joint ventures with regional partners to maintain market access and control over critical supply elements. Meanwhile, payers and health systems could increase emphasis on total cost of care analyses that capture the long-term implications of device selection under a tariff-inflated procurement environment.
While the immediate effect centers on cost and sourcing, the cumulative impact extends to innovation pipelines and market structure. Firms that proactively redesign supply chains, enhance cost transparency, and engage clinicians with evidence that demonstrates sustained value will be better positioned to navigate this evolving policy landscape.
Holistic segmentation analysis that connects product variants, end user behaviors, clinical applications, material science, and distribution pathways to strategic opportunity
Segmentation-informed insights that reveal where clinical demand, product innovation, and distribution dynamics intersect for endoprosthesis stakeholders
Analyzing the market through product type lenses highlights differentiated clinical pathways and engineering priorities. Dental implants, comprising fixed and removable solutions, demand precision in osseointegration and prosthetic interfaces, whereas hip implants-spanning partial, revision, and total designs-require attention to biomechanics and modularity for revision scenarios. Knee implants divided into primary and revision categories emphasize balancing kinematics with longevity, while shoulder offerings such as hemi, reverse, and total configurations reflect divergent indications and surgical techniques. Spinal systems across cervical, lumbar, and thoracic segments prioritize stability, fusion biology, and compatibility with minimally invasive approaches. These product-specific dynamics influence R&D focus, clinical training, and inventory planning in distinct ways.End user segmentation shapes procurement behavior and product requirements. Ambulatory surgical centers, whether free-standing or hospital-owned, often prioritize implants and instruments that reduce operative time and simplify sterilization, while hospitals-both private and public-balance breadth of product portfolios with negotiated purchasing agreements. Orthopedic clinics, including chain and independent models, emphasize clinician preference and streamlined stocking practices. These end user differences underscore the need for flexible commercial strategies that accommodate divergent procurement cycles, surgical volumes, and operational constraints.
Application-based segmentation underscores cross-disciplinary opportunity areas. Cardiovascular devices such as grafts, heart valves, and vascular stents require rigorous hemodynamic performance and biocompatibility, whereas dental bridges and implants focus on load distribution and soft tissue integration. Neuro applications like clips and shunts prioritize durability and minimal inflammatory response, and orthopedic applications-joint replacement, spinal fusion, and trauma-demand robust mechanical behavior and proven fixation approaches. Recognizing these application-specific requirements enables manufacturers to tailor materials, surface treatments, and clinical evidence packages appropriately.
Material choices further stratify product strategies, with ceramics, cobalt-chrome in porous and solid forms, polymers, titanium, and ultra-high-molecular-weight polyethylene each offering distinct trade-offs between strength, wear resistance, and biologic integration. Distribution channels ranging from direct sales to distributor networks and online platforms shape market access, sales force configurations, and service models. Integrating insights across these segmentation dimensions reveals where clinical need, material science, and distribution efficiency converge to create differentiated value propositions for providers and patients.
Strategic regional intelligence outlining how Americas, Europe, Middle East & Africa, and Asia-Pacific markets diverge in regulation, adoption, and supply chain priorities
Regional dynamics and strategic considerations across major global markets that influence investment, clinical adoption, and supply chain design for endoprostheses
In the Americas, care delivery systems and procurement practices vary between private and public providers, with a persistent focus on outcome-driven purchasing and an appetite for novel implants that demonstrably improve recovery and reduce revision rates. North American clinical networks favor evidence generation and adoption pathways that incorporate real-world data and comparative effectiveness research, while Latin American markets often emphasize cost-efficient solutions and partnerships that enable technology transfer and localized support.Europe, the Middle East & Africa present a mosaic of regulatory environments and reimbursement frameworks that influence product entry strategies. Western European markets frequently require rigorous clinical evidence and post-market surveillance commitments, whereas emerging markets in the region prioritize access and affordability, sometimes through public procurement mechanisms. Across the Middle East and Africa, infrastructure disparities necessitate adaptable device designs and service models that address variability in surgical capacity and supply logistics.
Asia-Pacific encompasses mature markets with advanced surgical capabilities alongside rapidly developing healthcare systems experiencing rising elective procedure volumes. In several markets, government initiatives aim to increase domestic manufacturing capacity and secure supply chain resilience, which affects partnership models and local regulatory navigation. Clinically, surgeons in the region demonstrate strong interest in cost-effective innovations that do not compromise on long-term outcomes, creating opportunities for modular solutions and scalable manufacturing approaches.
Understanding these regional distinctions is critical for aligning regulatory pathways, KOL engagement, and supply chain investments. Manufacturers and providers must tailor evidence generation, pricing strategies, and market access plans to regional priorities while preserving global coherence in product quality and clinical messaging.
Competitive behaviors and collaborative strategies among leading firms that dictate innovation focus, clinical partnerships, and consolidation patterns
Competitive and collaborative behaviours among leading companies that influence innovation trajectories, clinical partnerships, and market consolidation dynamics
Key companies in the endoprosthesis arena are pursuing differentiated strategies across R&D intensity, vertical integration, and clinical engagement. Some firms prioritize materials science and proprietary surface technologies to secure durable clinical advantages, while others emphasize systems-level solutions that integrate implants with instrumentation, digital planning tools, and aftercare services. These strategic choices affect partnership formation, acquisition activity, and the nature of post-market studies aimed at demonstrating comparative performance.Collaborative models have become increasingly important. Strategic alliances with academic centers, surgical societies, and technology providers accelerate clinical validation and facilitate the adoption of novel devices. At the same time, selective mergers and acquisitions enable companies to broaden product portfolios, streamline distribution footprints, and capture synergies in manufacturing. Investors and corporate development teams are thus evaluating targets not only for product fit but for their capacity to contribute to integrated, service-oriented offerings.
Moreover, companies that excel in clinician-centric training and support-delivering comprehensive surgical education, simulation tools, and evidence dissemination-tend to achieve deeper market penetration. These organizations combine robust clinical evidence with effective field engagement to shift long-standing procurement preferences. As competition intensifies, the balance between proprietary innovation and open collaborative ecosystems will determine which firms achieve sustainable differentiation.
Targeted, operational, and clinical strategies leaders should adopt to strengthen product differentiation, supply resilience, and market access success
Actionable recommendations for industry leaders to align product strategy, clinical evidence, and commercial operations with evolving market realities
Leaders should prioritize integrated product roadmaps that align materials innovation with modularity and ease of use to meet the needs of diverse clinical settings. Investments in surface technologies, porous architectures, and designs that streamline revision procedures will address both clinician preferences and long-term outcome expectations. In addition, firms must embed compatibility with digital surgical ecosystems into product planning to enhance intraoperative reproducibility and postoperative data capture.Supply chain resilience requires proactive measures, including diversified sourcing, targeted onshoring for critical components, and collaborative contracts with logistics partners to reduce lead-time risk. Concurrently, companies should strengthen value communication by generating real-world evidence and health economic analyses that articulate total care benefits rather than only unit-level cost metrics. These efforts will support negotiations with payers and purchasing groups under increasingly value-oriented procurement frameworks.
Commercial teams need to tailor engagement models to end user segmentation, offering streamlined instrument sets for ambulatory centers and comprehensive service packages for hospitals and clinics. Training programs that combine hands-on surgical education with outcomes data will accelerate clinician adoption. Finally, corporate development strategies should target partnerships and acquisitions that extend product portfolios into complementary applications, while preserving clinical credibility through rigorous evidence generation.
Transparent description of the research approach, data sources, and analytical methods employed to generate actionable and verifiable insights on endoprosthesis markets
Methodological approach and data synthesis techniques used to compile a rigorous, evidence-driven perspective on the endoprosthesis landscape
This research synthesizes peer-reviewed clinical literature, public regulatory filings, surgical society guidelines, and primary interviews with practicing surgeons, procurement leaders, and industry experts. Data triangulation ensures that insights reflect clinical realities, technological capabilities, and commercial practices across diverse care settings. Qualitative inputs from key opinion leaders were used to contextualize device performance against procedural workflows and patient-reported outcomes.Analytical methods included systematic appraisal of material science developments, comparative assessment of device design trade-offs, and evaluation of distribution channel effectiveness based on provider purchasing behaviors. Supply chain analysis integrated supplier concentration metrics, lead-time sensitivity, and regional manufacturing capabilities to highlight sources of operational risk. Additionally, scenario analysis explored potential responses to policy shifts, including tariff changes and evolving reimbursement models, to inform strategic recommendations.
Throughout, emphasis was placed on transparency of assumptions and reproducibility of analytical steps. Findings were validated through iterative review with clinical experts and commercial stakeholders to ensure that conclusions are actionable and grounded in observable trends rather than extrapolated forecasts.
Concise synthesis of strategic imperatives that integrate clinical excellence, operational resilience, and value articulation for sustained advantage
Conclusion synthesizing the strategic imperatives for stakeholders seeking durable competitive advantage in the endoprosthesis arena
The endoprosthesis landscape is defined by converging pressures from materials innovation, digital integration, changing care settings, and evolving procurement expectations. Stakeholders who anticipate these convergences and align product design, evidence generation, and supply chain strategy will capture clinical preference and mitigate operational risk. In particular, prioritizing implants that balance advanced biologic integration with modularity and ease of use will meet the dual demands of surgeons and ambulatory centers alike.Moreover, proactive management of sourcing and manufacturing footprints in light of policy uncertainties will preserve market access and protect margin performance. Companies that combine rigorous, real-world evidence with clinician-focused education and adaptable commercial models will secure longer-term adoption and create barriers to commoditization. Finally, regional nuance matters: tailoring regulatory strategies, pricing, and partnership models to local priorities will accelerate market entry and sustain growth.
Taken together, the strategic imperative is clear: integrate clinical excellence with operational resilience and compelling value communication to succeed in a rapidly evolving endoprosthesis environment.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Endoprosthesis Market
Companies Mentioned
- ConforMIS, Inc.
- DePuy Synthes, Inc.
- DJO Global, Inc.
- Exactech, Inc.
- Globus Medical
- LimaCorporate S.p.A.
- Medacta International S.A.
- Medtronic plc
- MicroPort Scientific Corporation
- Smith & Nephew plc
- Stryker Corporation
- Zimmer Biomet Holdings, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 186 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
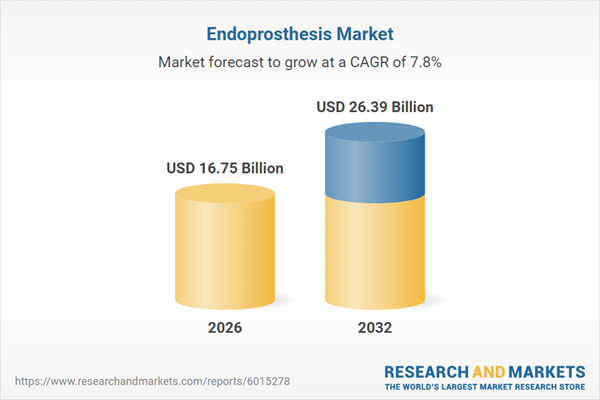
| Estimated Market Value ( USD | $ 16.75 Billion |
| Forecasted Market Value ( USD | $ 26.39 Billion |
| Compound Annual Growth Rate | 7.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |